A kind of metallic lithium alloy and its preparation method and application
A metal lithium and alloy technology, applied in structural parts, electrochemical generators, negative electrodes, etc., can solve the problems of inability to large-scale production, limit industrial application, and stay, and achieve stable circulation and excellent lithium ion transport capacity. and mechanical properties, the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The preparation method of metal lithium alloy provided by the invention comprises the following steps:
[0037] 1) Extracting alkali metal salt solids from lithium ore leachate or purified lithium brine;
[0038] Wherein, the alkali metal salt solids include lithium salt solids, sodium salt solids, potassium salt solids, rubidium salt solids and cesium salt solids;
[0039] 2) heating the alkali metal salt solid obtained in step 1) under an inert gas until it completely melts;
[0040] 3) Melting and electrolyzing the melted alkali metal salt solid under inert gas for 1-10 hours to obtain metal lithium alloy;
[0041] Among them, the electrolytic current density is 1 ~ 50mA / cm 2 , The electrolysis temperature is 400 ~ 850 ℃.
[0042] The concentration ratio of lithium / magnesium and lithium to boron in the purified lithium brine or lithium ore leaching solution in step 1) is greater than 1, and the alkali metal content is greater than 10 mg / L.
[0043] The preparatio...
Embodiment 1
[0047] Add a sufficient amount of 5 mol / L HTFSI solution and 3 mol / L (t-BAMBP) solvent to the concentrated brine of Qinghai Xitai Jinel Salt Lake concentrated brine after purification as 1,3-dioxolane (DOL), and separate to obtain Add 3mol / L hydrochloric acid to the organic solution containing LiTFSI and other alkali metal salts, back-extract the alkali metals to obtain the corresponding alkali metal chloride salt solution, and evaporate the water to obtain the corresponding alkali metal chloride salt solid.
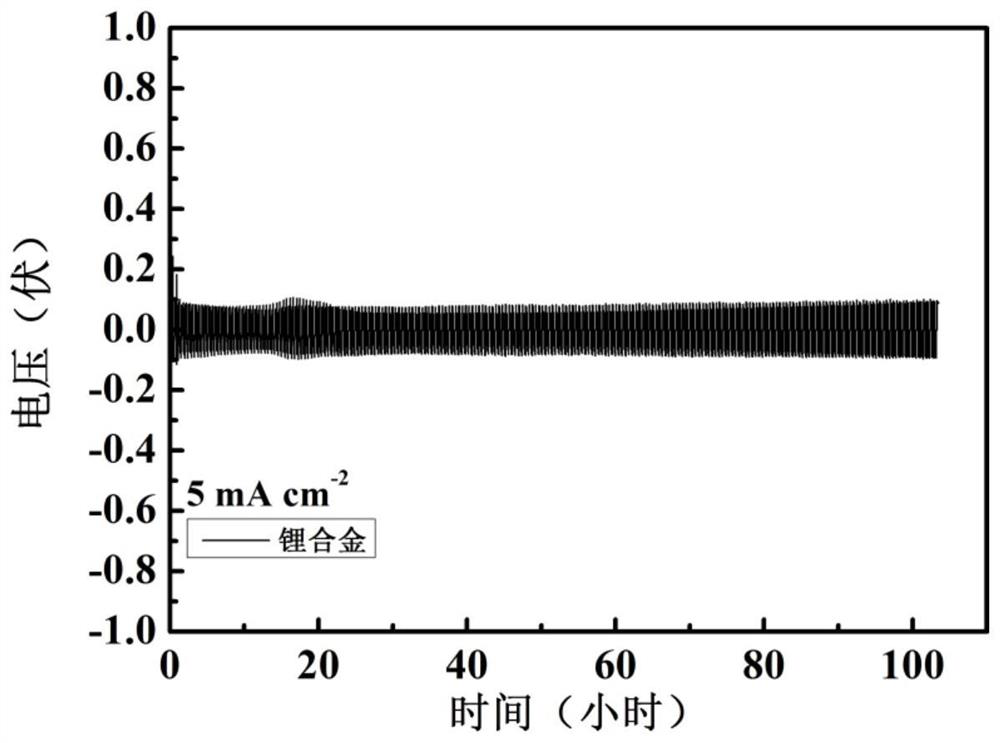
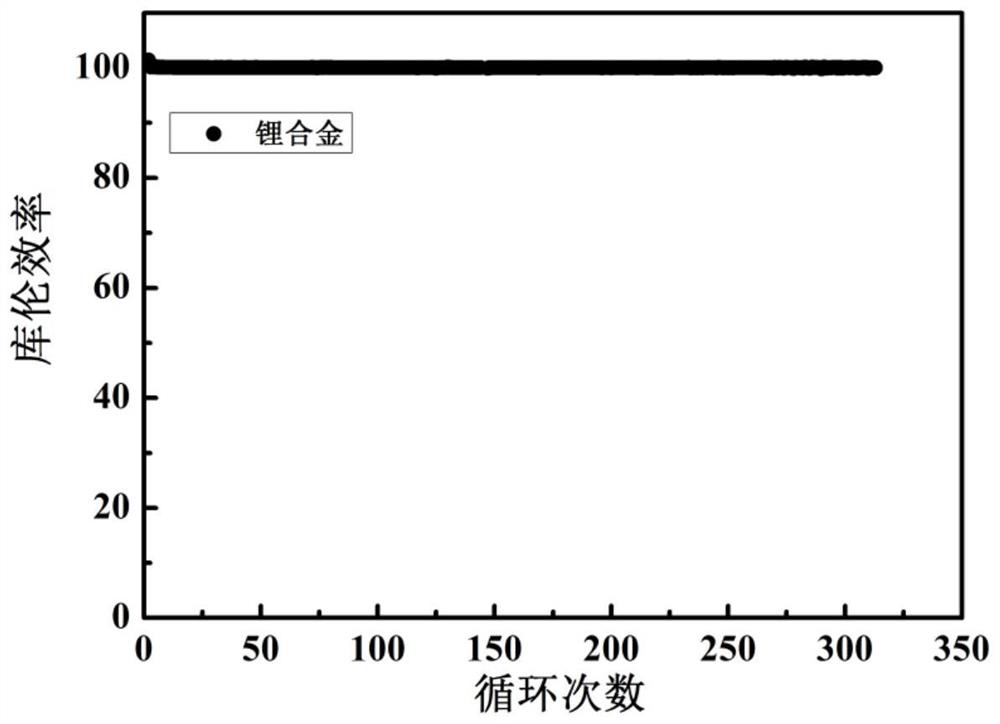
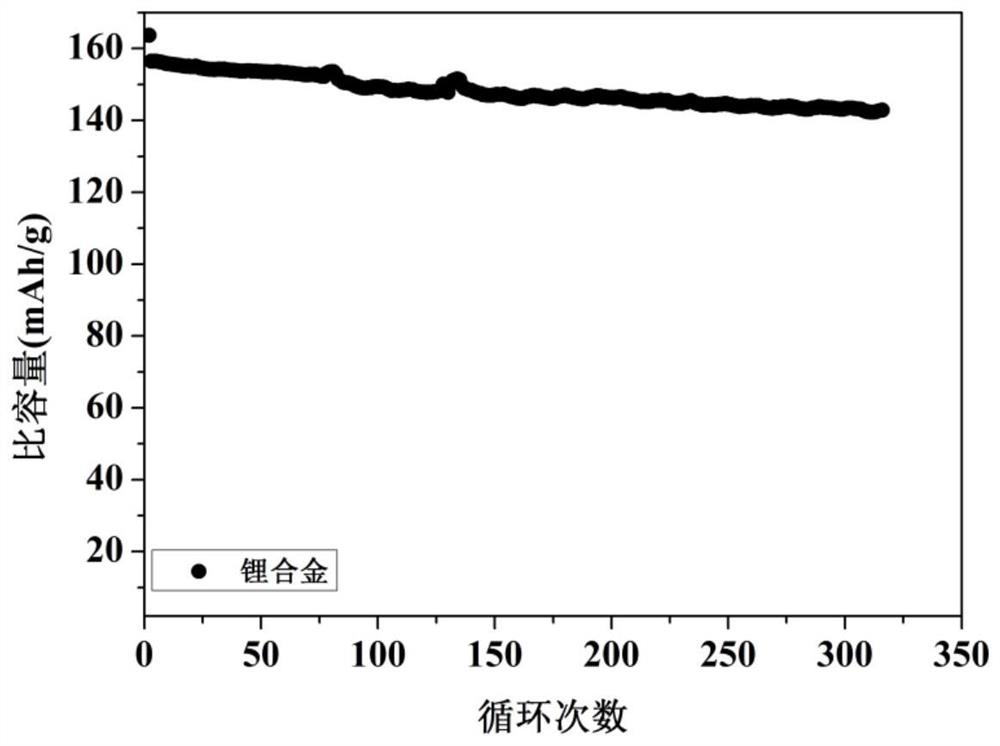
[0048] Mix the above alkali metal salt chloride solids evenly, add them into the electrolytic cell, and heat until the solids are completely melted. The temperature of the electrolytic cell is constant at 400°C, the cathode potential is controlled at -4.1V, and the constant DC current density is 50mA / cm 2 The electrolysis was carried out for 10 hours at low temperature, and lithium, sodium, potassium, rubidium and cesium were collected. Cool to obtain the lithium alloy,...
Embodiment 2
[0051] Take 5L of a certain lepidolite leach solution after purification, add a sufficient amount of 6mol / L HClO 4 Solution, and the s-BAMBP of 1mol / L, the solvent is ethylene glycol dimethyl ether (DME), carry out 5 stages of countercurrent extraction. LiClO-containing 4 and other organic solutions of alkali metal salts, and then add 8 mol / L hydrochloric acid to obtain an alkali metal chloride salt solution, evaporate water, and dry to obtain lithium, sodium, potassium, rubidium, and cesium chloride solids.
[0052] Mix the above alkali metal salts evenly, add them into the electrolytic cell, and heat until the solids are completely melted. The whole system is in an argon atmosphere. Stabilize the above electrolyte to 850°C, the cathode potential is constant at -3.5V, at a constant DC current of 1mA / cm 2 electrolysis for 1 h. Lithium, sodium, potassium, rubidium, and cesium were collected, cooled to obtain a lithium alloy with a purity of 98.4%, and the obtained lithium a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com