Preparation facilitating wound healing and preparation method of preparation
A wound healing and penetration enhancer technology, applied in the field of medicine, can solve the problems of low solubility of tofacitinib, difficult to achieve therapeutic effect, and aggravate wound infection, so as to promote drug absorption, prevent bacteria from entering the wound surface, and prevent wound infection. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
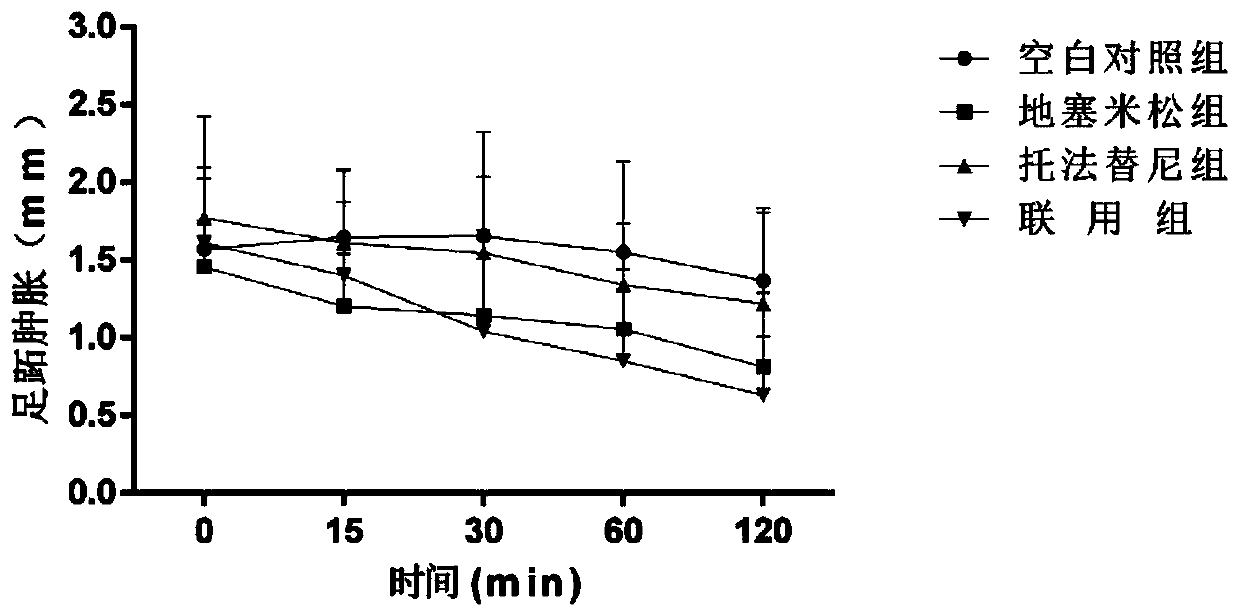
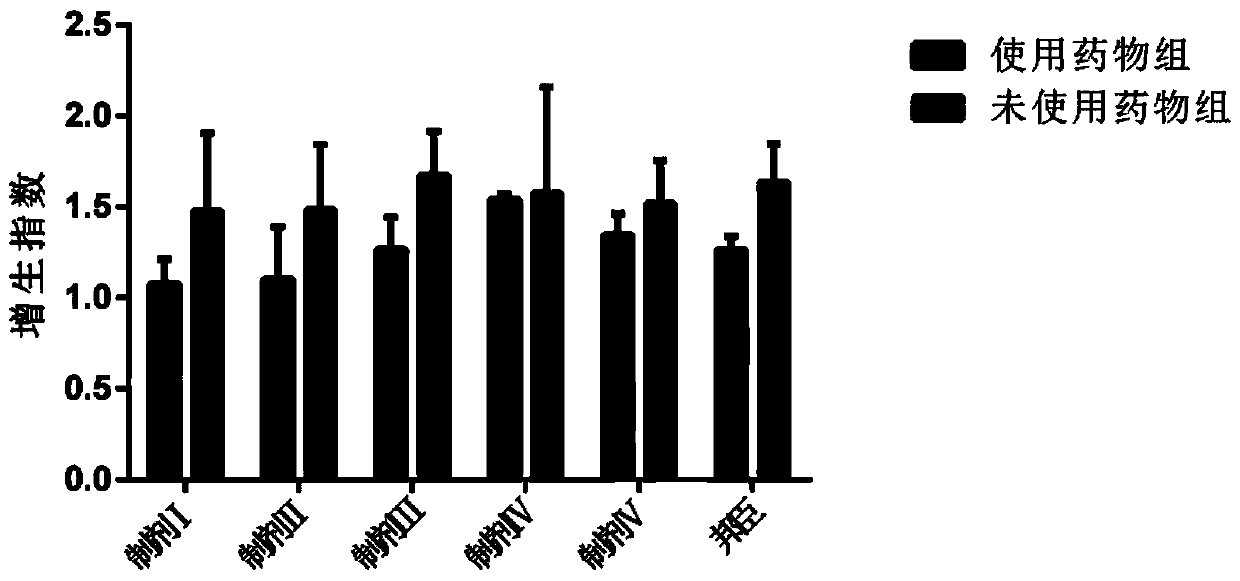
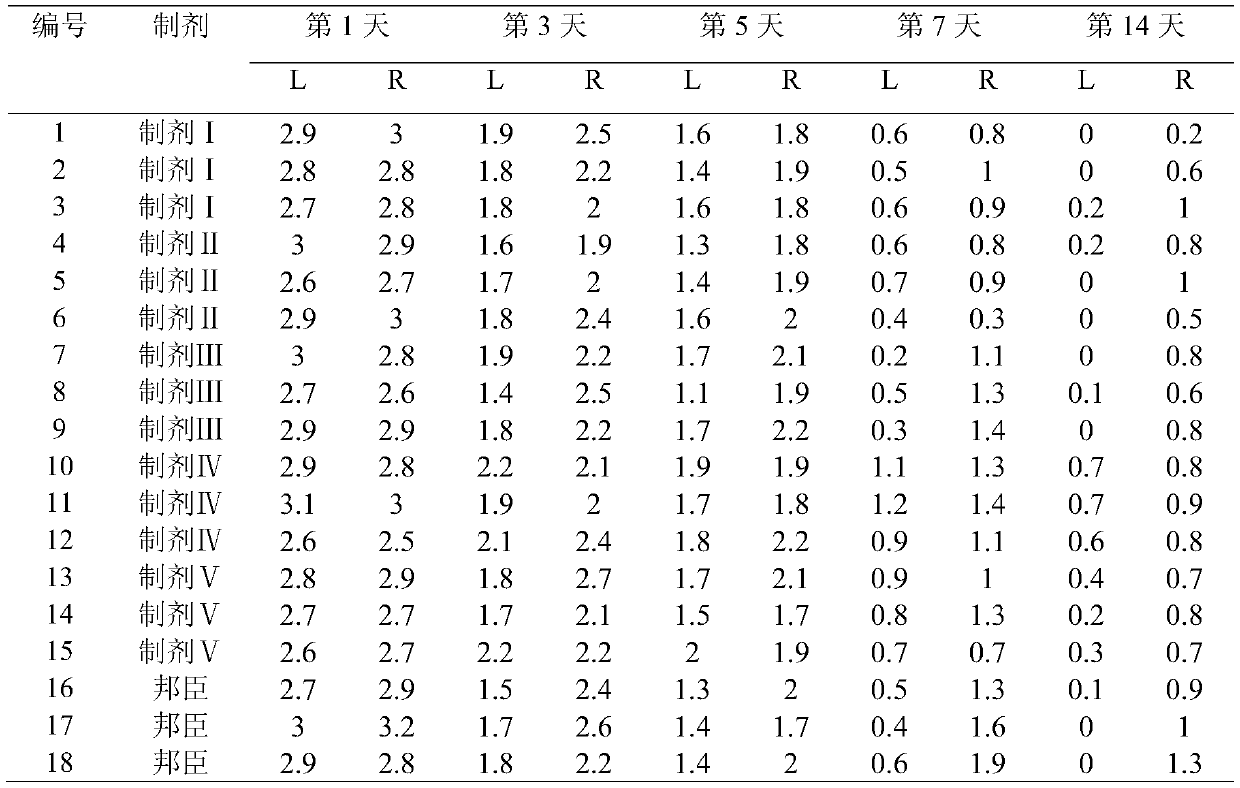
Image
Examples
Embodiment 1
[0028] A preparation for promoting wound healing, by weight percentage, the preparation comprises the following components: viscosity is 50mPa·s, molecular weight is 15% of polydimethylsiloxane 3800, tofacitinib 0.2%, corticosteroid ( Dexamethasone) 1.5%, acceptable excipients in external skin preparations [moisturizing agent (glycerol) 5%, emollient (palm oil) 6%, penetration enhancer (isopropyl myristate) 2%, Antioxidant (tocopherol) 2%] 15%, balance is purified water. The preparation is prepared as follows:
[0029] (1) According to the weight percent of each component in the above-mentioned preparation, take by weighing viscosity is 50mPa·s, molecular weight is polydimethylsiloxane, tofacitinib, corticosteroid (dexamethasone), humectant ( glycerol), emollient (palm oil), penetration enhancer (isopropyl myristate), antioxidant (tocopherol) and purified water;
[0030] (2) dissolving tofacitinib, corticosteroid, humectant, emollient and penetration enhancer in purified wat...
Embodiment 2
[0033] A preparation for promoting wound healing, by weight percentage, the preparation comprises the following components: viscosity is 100mPa·s, molecular weight is 20% of polydimethylsiloxane 6000, tofacitinib 0.3%, corticosteroid ( triamcinolone acetonide) 1.0%, acceptable excipients in external skin preparations [stabilizer (carboxymethylcellulose) 2%, humectant (polyethylene glycol) 9%, emollient (caprylic capric triglyceride) 8%, penetration enhancer (PEG-200) 3%, antioxidant (ascorbic acid) 3%] 25%, and the balance is purified water. The preparation is prepared as follows:
[0034] (1) According to the weight percentage of each component in the above preparation, it is said that polydimethylsiloxane, tofacitinib, corticosteroid (triamcinolone acetonide), stabilizer (carboxylate) with a viscosity of 100 mPa·s and a molecular weight of 6000 methylcellulose), humectant (polyethylene glycol), emollient (caprylic capric triglyceride), penetration enhancer (PEG-200), antiox...
Embodiment 3
[0038] A preparation for promoting wound healing, by weight percentage, the preparation comprises the following components: viscosity is 30mPa·s, molecular weight is 10% of polydimethylsiloxane 3000, tofacitinib 0.2%, corticosteroid ( Budesonide) 1.7%, acceptable excipients in external skin preparations [stabilizer (xanthan gum) 3%, humectant (1,3-butanediol) 8%, emollient (lanolin) 5%, Penetration enhancer (laurocapram) 3%, antioxidant (sodium sulfite) 5%] 24%, and the balance is purified water. The preparation is prepared as follows:
[0039] (1) According to the percentage by weight of each component in the above-mentioned preparation, take the polydimethylsiloxane, tofacitinib, corticosteroid (budesonide), stabilizer ( xanthan gum), humectant (1,3-butanediol), emollient (lanolin), penetration enhancer (laurozone), antioxidant (sodium sulfite) and purified water;
[0040] (2) dissolving tofacitinib, corticosteroid, stabilizer, humectant, emollient and penetration enhancer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com