Control method for oxidation prevention of vitamin C tablet
A control method and vitamin technology, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pill delivery, etc. It can solve the problems of shortened shelf life, exposed tablets, and high production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The preparation method of vitamin C microcapsules comprises the following steps: (1) Weighing a certain amount of polyglycerol fatty acid ester and hardened vegetable oil into water, heating and stirring in a water bath at 45° C. for 8 minutes, so that the polyglyceryl fatty acid ester and hardened vegetable oil Evenly dispersed, wherein, the ratio of polyglycerol fatty acid ester, hardened vegetable oil and water is 1:1:(15-20)(W / V), and the wall material solution is obtained; (2) Weigh 40-50 parts of vitamin C , 0.05-2 parts of citric acid, 0.05-2 parts of malic acid, 0.05-0.1 part of cyclohexanediaminetetraacetic acid, and quickly add it to water, and stir to obtain a core material solution; (3) according to the core material solution and The mass ratio of the wall material solution is 1:(5-15). Add the core material solution into the wall material solution, stir and mix evenly, and emulsify at a speed of 2200r / min for 3min; (4) emulsify the solution in step 3 Vitami...
Embodiment 1
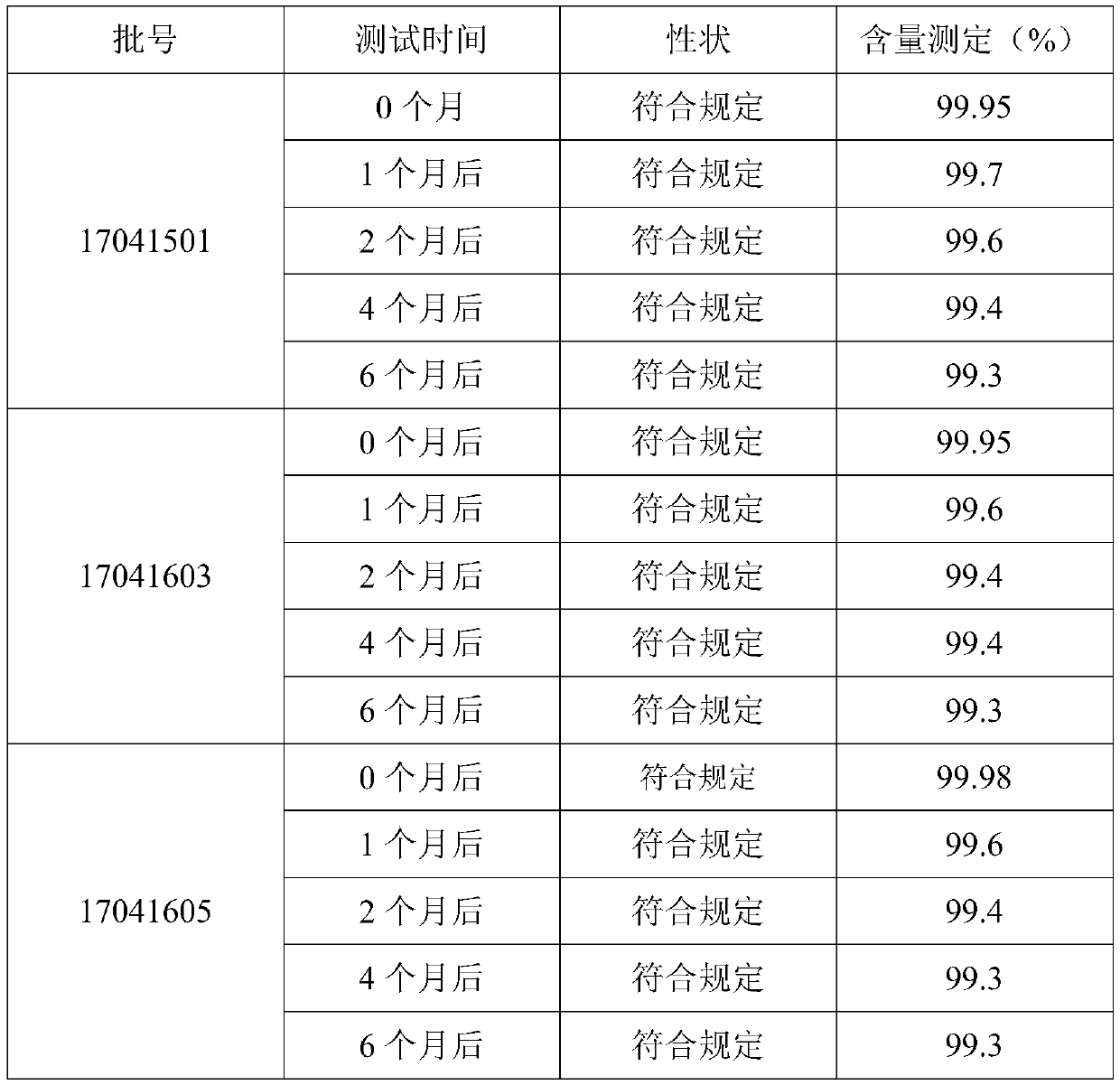
[0021] A method for controlling oxidation resistance of vitamin C tablets. The production process of vitamin C tablets includes the following steps: S1: preparing vitamin C microcapsules; S2: weighing the following raw materials in parts by weight: 70 parts of vitamin C microcapsules, ring 0.002 part of hexanediaminetetraacetic acid, 8 parts of pregelatinized starch, 15 parts of lactose-starch complex, 0.0.5 part of histidine, 0.05 part of lysine and 1 part of benzoic acid, and crush each raw material for 60 Mesh sieve; S3: mix all the sieved raw materials evenly to obtain a mixed material; S4: directly compress the mixed material under a certain tabletting environment. Raw materials and process parameter control: the weight ratio of lactose and starch in the lactose-starch compound is 1:0.175, the water mass ratio of the mixed material in step S3 is 6%, and the bulk density of the mixed material is 0.35g / ml; The sheet environment is: a humidity range of 20% RH, and a temperat...
Embodiment 2
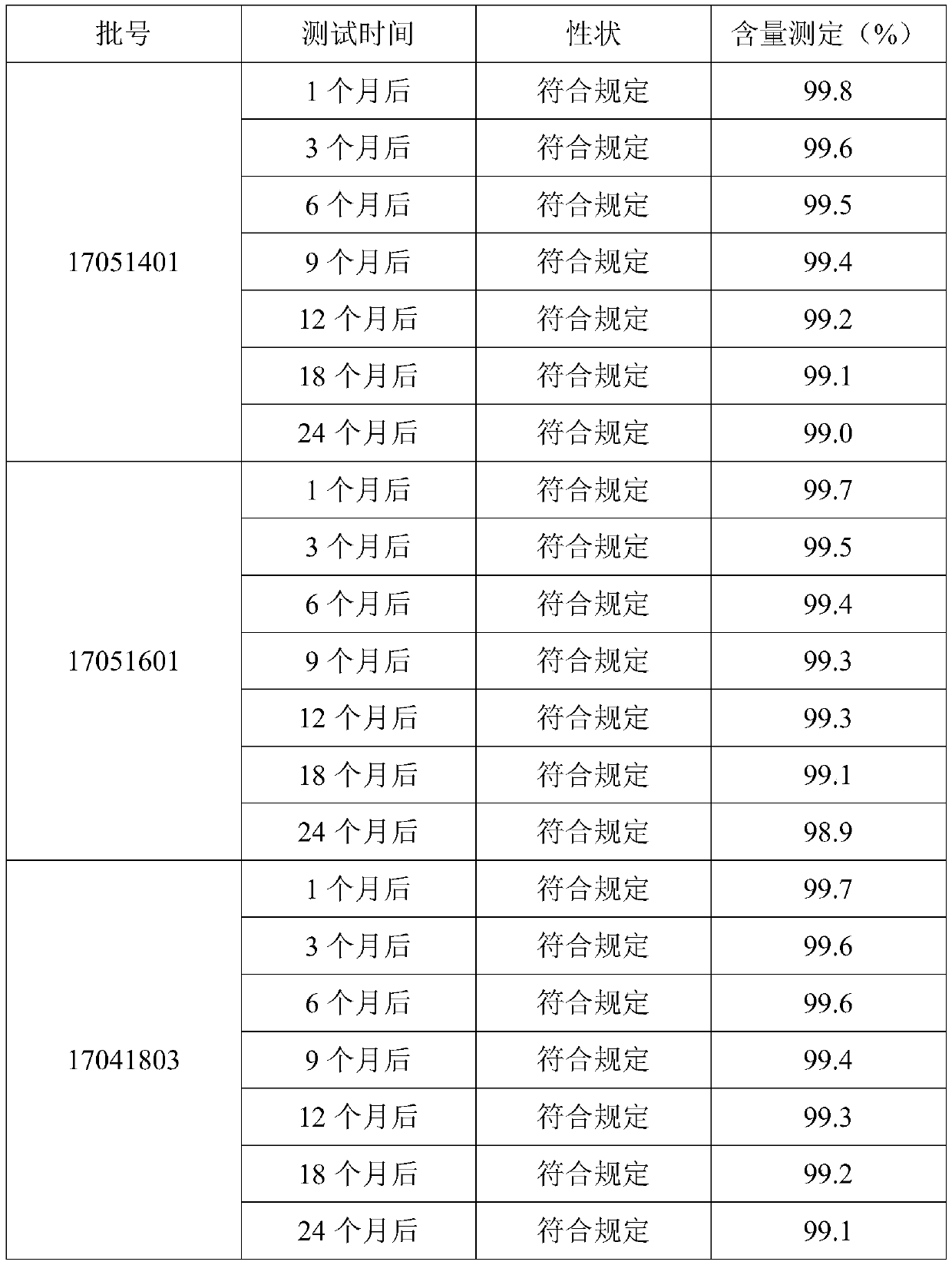
[0025] A method for controlling oxidation resistance of vitamin C tablets. The production process of vitamin C tablets includes the following steps: S1: preparing vitamin C microcapsules; S2: weighing the following raw materials in parts by weight: 85 parts of vitamin C microcapsules, cyclohexane 0.15 parts of diaminetetraacetic acid, 10 parts of pregelatinized starch, 25 parts of lactose-starch complex, 0.1 part of histidine, 0.1 part of lysine, 1 part of citric acid, and 0.1 part of malic acid. Crushing through a 60-mesh sieve; S3: mixing all the sieved raw materials evenly to obtain a mixed material; S4: directly compressing the mixed material under a certain tabletting environment. Raw materials and process parameter control: the weight ratio of lactose and starch in the lactose-starch compound is 1:0.175, the water mass ratio of the mixed material in step S3 is 5%, and the bulk density of the mixed material is 0.65g / ml; The sheet environment is: the humidity range is 40RH...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com