Rheum officinale free anthraquinone self-microemulsion tablet and preparation method thereof
A free anthraquinone, self-micro-emulsifying technology, used in the digestive system, pharmaceutical formulations, emulsion delivery, etc., can solve the problems of free anthraquinone prone to polymerization, difficult to dissolve gastrointestinal fluid, and emulsion droplet aggregation, etc. The effect of drug onset time, production cost reduction and stability improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation of embodiment 1 rhubarb free anthraquinone self-microemulsion tablet
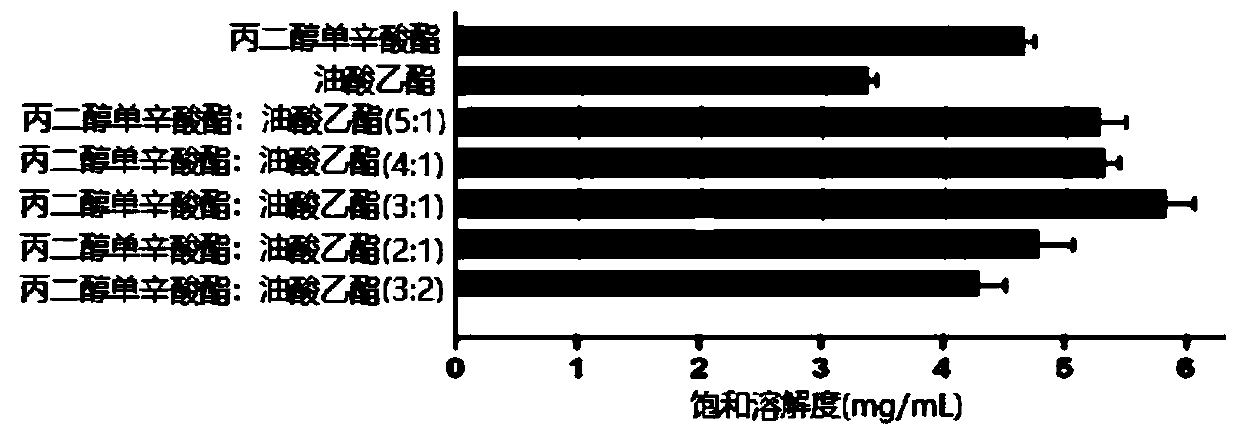
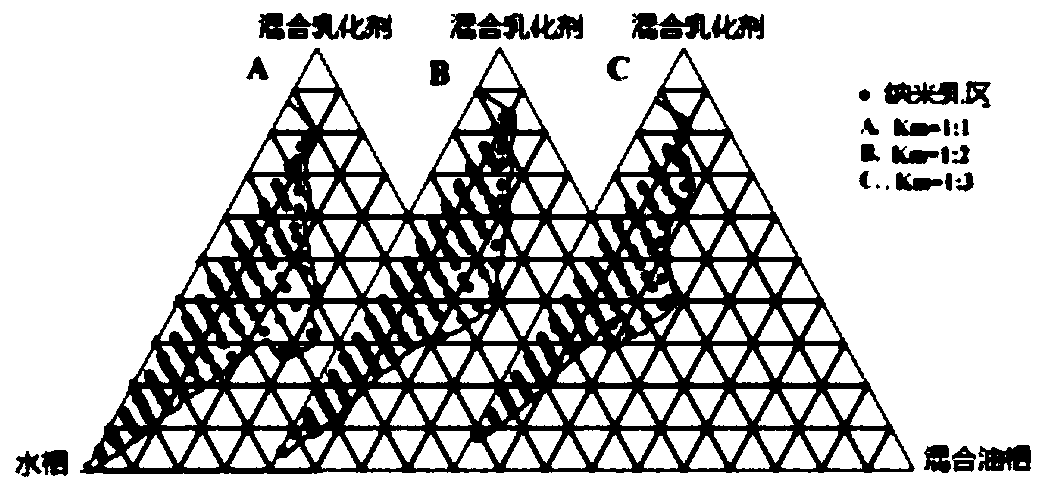
[0026] (1) Preparation of concentrated rhubarb free anthraquinone nanoemulsion: take the prescribed amount of polyoxyethylene hydrogenated castor oil (emulsifier), heat and melt in a water bath at 35°C, and add co-emulsifier according to 1:1 (w / w) Diethylene glycol monoethyl ether (co-emulsifier), stirred evenly at room temperature to obtain a mixed emulsifier; uniformly mixed propylene glycol monocaprylate and ethyl oleate at a ratio of 3:1 (w / w), and added Rhubarb free anthraquinone so that its concentration is 5.1 mg / mL to obtain a mixed oil phase. Mix the mixed oil phase and the mixed emulsifier at a ratio of 31.45:68.55 (w / w), pay attention not to add water to the system, and gently stir for 15 minutes to obtain a clear and uniform concentrated rhubarb free anthraquinone nanoemulsion.
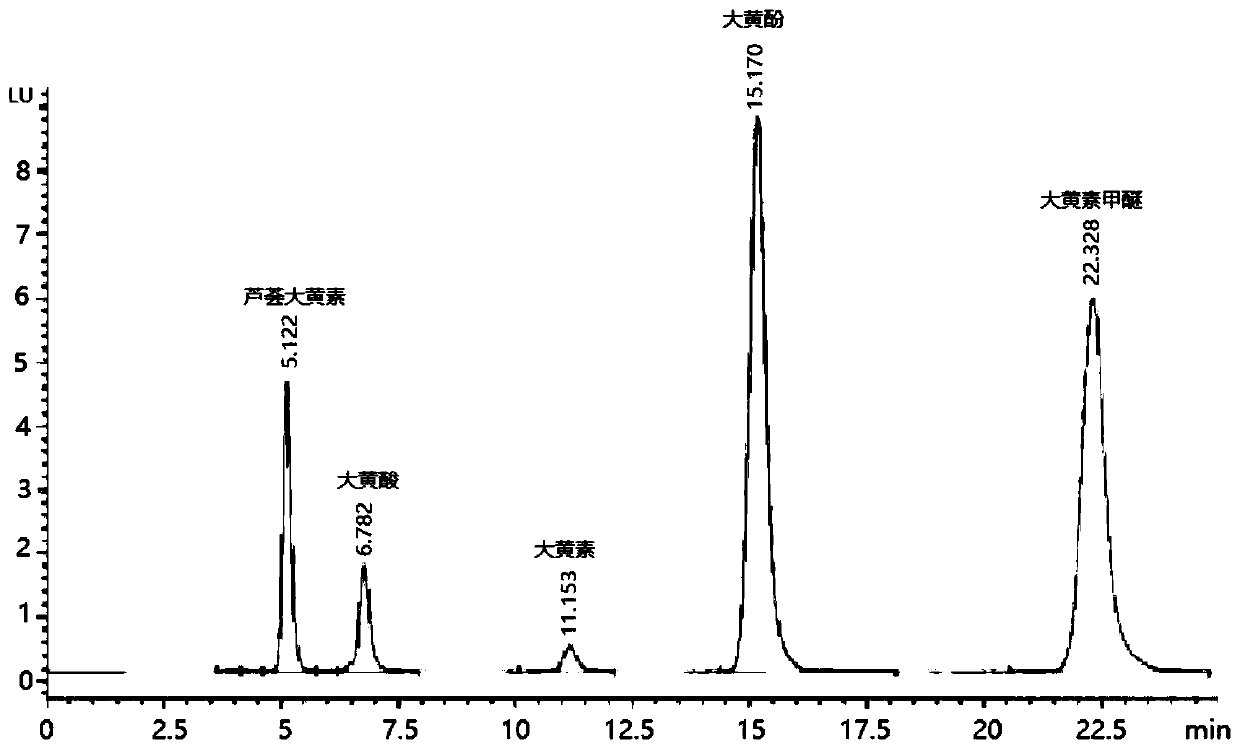
[0027] figure 1 It is the HPLC chromatogram of rhubarb free anthraquinone used in the presen...
Embodiment 2
[0039] Embodiment 2 self-emulsification of rhubarb free anthraquinone self-microemulsification tablet in water
[0040] Rhubarb free anthraquinone self-microemulsifying tablet disintegrates and dissolves in water, artificial gastric juice or intestinal juice, and the emulsion droplets after self-emulsification are spherical, with a particle size between 20-100nm (see Figure 5 ).
[0041] The morphology of emulsion droplet after emulsification of rhubarb free anthraquinone from microemulsion sheet was observed by transmission electron microscope. Put rhubarb free anthraquinone self-microemulsified tablet in distilled water at 37±0.5°C, stir at 100rpm for 30min to completely disperse the tablet in purified water, and filter the resulting suspension with a 0.45μm organic microporous membrane. Drop the filtrate on the copper grid (common carbon coating), and then dye it with 2.0% phosphotungstic acid, and dry the coated grid at room temperature. The electron microscope shows tha...
Embodiment 3
[0043] Example 3 The in vitro release of rhubarb free anthraquinone from microemulsified tablets
[0044] Rhubarb free anthraquinone powder is directly mixed with aluminum magnesium silicate, cross-linked polyvinylpyrrolidone and magnesium stearate, and dry-pressed to obtain rhubarb free anthraquinone ordinary tablets (control sample); Anthraquinone self-microemulsifying tablet (test sample). In vitro release tests were carried out in accordance with the relevant regulations of the 2015 edition of the Chinese Pharmacopoeia.
[0045] The release rate of rhubarb free anthraquinone from rhubarb free anthraquinone self-microemulsified tablets and rhubarb free anthraquinone ordinary tablets was determined by small cup method using RC-8D dissolution tester. The speed of the stirring paddle of the dissolution apparatus is 100rpm, the temperature of the water bath is 37±0.5°C, and the release media are respectively pH 1.2 hydrochloric acid solution (artificial gastric juice) and pH 6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com