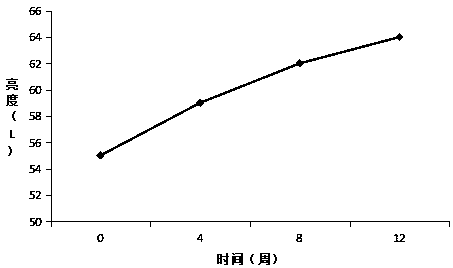
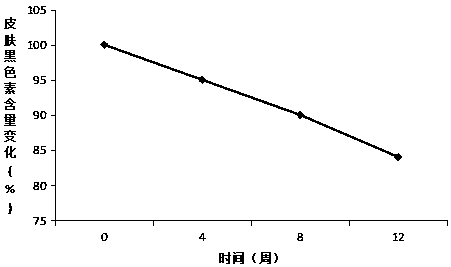
Whitening buccal tablets containing SOD components
A component and whitening technology, which is applied in the field of whitening lozenges with SOD components, can solve the problems of accelerating melanin metabolism and insufficient effect, and can reduce the formation of dopa and dopaquinone substances, with remarkable effect and speed up the skin. The effect of update speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] SOD 0.5%, Resveratrol 5%, Niacinamide 5%, Vitamin C 5%, Starch Slurry 12%, Magnesium Stearate 2%, Microcrystalline Cellulose 40%, Mannitol 30.5%. The dissolution of this embodiment is 15.30min.
[0043] Preparation includes the following steps:
[0044] Step 1: Weigh SOD, resveratrol, nicotinamide, vitamin C, microcrystalline cellulose, and mannitol, pass through a 100-mesh sieve, and mix well;
[0045] Step 2: granulating, adding starch slurry to make soft material, and granulating through a 20-mesh sieve;
[0046] Step 3: drying, the drying temperature is 30°C;
[0047] Step 4: granulation and tabletting. After granulation, in order to make the surface of the buccal tablet smooth and easier to release from the mold, add magnesium stearate, mix evenly, and send it to a tablet machine for tableting;
[0048] Step 4: Sterilization and packaging, irradiating the tablet under ultraviolet light for 15 minutes, and performing blister packaging.
Embodiment 2
[0050] Formula SOD 0.6%, Resveratrol 6%, Niacinamide 6%, Vitamin C 6%, Starch Slurry 11%, Talc 1.4%, Microcrystalline Cellulose 37%, Sorbitol 32%. The dissolving time of the present embodiment is 19.53min.
[0051] Preparation includes the following steps:
[0052] Step 1: Weigh SOD, resveratrol, nicotinamide, vitamin C, microcrystalline cellulose, and sorbitol, pass through a 100-mesh sieve, and mix well;
[0053] Step 2: granulating, adding starch slurry to make soft material, and granulating through a 20-mesh sieve;
[0054] Step 3: drying, the drying temperature is 30°C;
[0055] Step 4: Sizing and tableting. After sizing, in order to make the surface of the buccal tablet smooth and easier to release from the mold, add talcum powder, mix evenly, and send it to a tablet machine for tableting;
[0056] Step 4: Sterilization and packaging, irradiating the tablet under ultraviolet light for 15 minutes, and performing blister packaging.
Embodiment 3
[0058] Formula: SOD 0.7%, Resveratrol 7%, Nicotinamide 7%, Vitamin C 7%, Starch Slurry 10%, Polyethylene Glycol-4000 1%, Microcrystalline Cellulose 38%, Glucose 29.3%. The dissolving time of the present embodiment is 17.25min.
[0059] Preparation includes the following steps:
[0060] Step 1: Weigh SOD, resveratrol, nicotinamide, vitamin C, microcrystalline cellulose, and glucose through a 100-mesh sieve and mix well;
[0061] Step 2: granulating, adding starch slurry to make soft material, and granulating through a 20-mesh sieve;
[0062] Step 3: drying, the drying temperature is 30°C;
[0063] Step 4: Sizing and tableting. After sizing, in order to make the surface of the buccal tablet smooth and easier to release from the mold, add polyethylene glycol-4000, mix evenly, and send it to a tablet machine for tableting;
[0064] Step 4: Sterilization and packaging, irradiating the tablet under ultraviolet light for 15 minutes, and performing blister packaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com