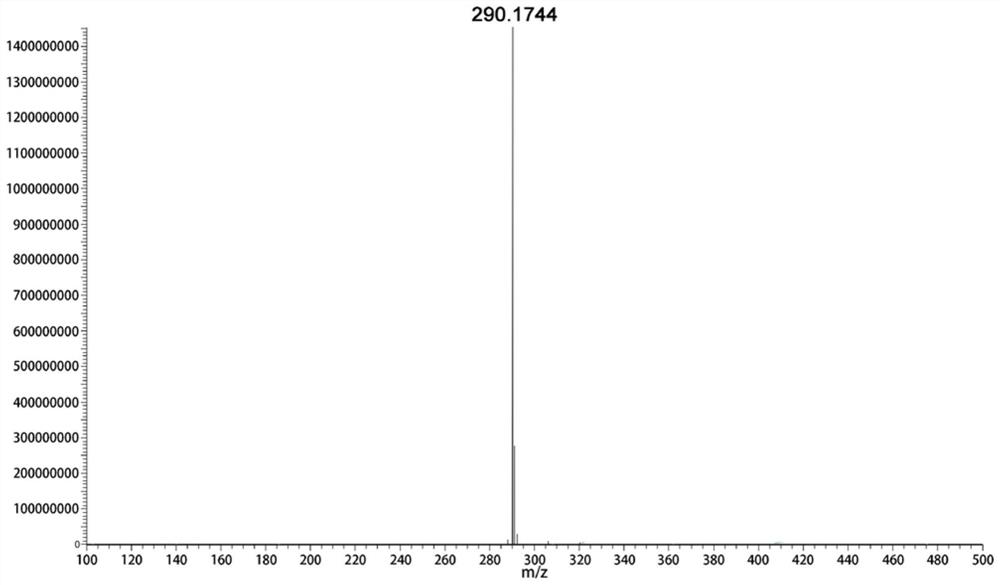
Hyospolamine reductase and its application
A technology of reductase and scopolaldehyde, applied in the directions of oxidoreductase, application, enzyme, etc., can solve the problems of inability to achieve total chemical synthesis, unclear biosynthetic pathway of secondary metabolites, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, cloning of hyoscyamine aldehyde reductase synthase (HAR) gene
[0034] (1) Extraction of total RNA from belladonna fibrous root
[0035] Take an appropriate amount of belladonna fibrous root tissue, grind it in liquid nitrogen, add it to a 1.5mL Eppendorf (EP) centrifuge tube filled with lysate, shake it fully, and extract total RNA according to the instructions of the TIANGEN kit. The quality of total RNA was identified by formaldehyde denaturing gel electrophoresis, and the RNA concentration was determined on a spectrophotometer.
[0036] (2) Cloning of HAR gene
[0037] Using the extracted total RNA as a template, synthesize cDNA according to the instructions of Tiangen FastKing cDNA First Strand Synthesis Kit; design HAR gene-specific primers, the specific primers are as follows:
[0038] HAR-F: 5'-atggattcttctggtgtcctct-3' (SEQ ID NO.1);
[0039] HAR-R: 5'-ttggttgctgctcaaacctag-3' (SEQ ID NO. 2).
[0040] The HAR gene was amplified from the total ...
Embodiment 2
[0041] Example 2, prokaryotic expression verification of the function of the HAR gene
[0042] (1) Prokaryotic expression and protein purification of HAR
[0043] The HAR gene was amplified by PCR, the restriction site EcoRI was introduced into the forward primer, and the restriction site SacI was introduced into the reverse primer. The complete sequence of the HAR coding region was connected to the plasmid pET28a by using the above two restriction sites to obtain the HAR prokaryotic expression vector pET28a-HAR. Primers are as follows:
[0044]EcoRI-HAR-F: 5'-cgcgaattcatggattcttctggtgtcctct-3' (SEQ ID NO.5);
[0045] XhoI-HAR-R: 5'-cgcctcgagctaggtttgagcagcaaccaa-3' (SEQ ID NO. 6).
[0046] The constructed pET28a-HAR plasmid was transformed into the prokaryotic expression strain BL21, and positive clones were screened by PCR to obtain the prokaryotic expression engineering strain BL21-pET28a-HAR. Take 100 μL of BL21-pET28a-HAR bacteria solution and inoculate it into 30 mL ...
Embodiment 3
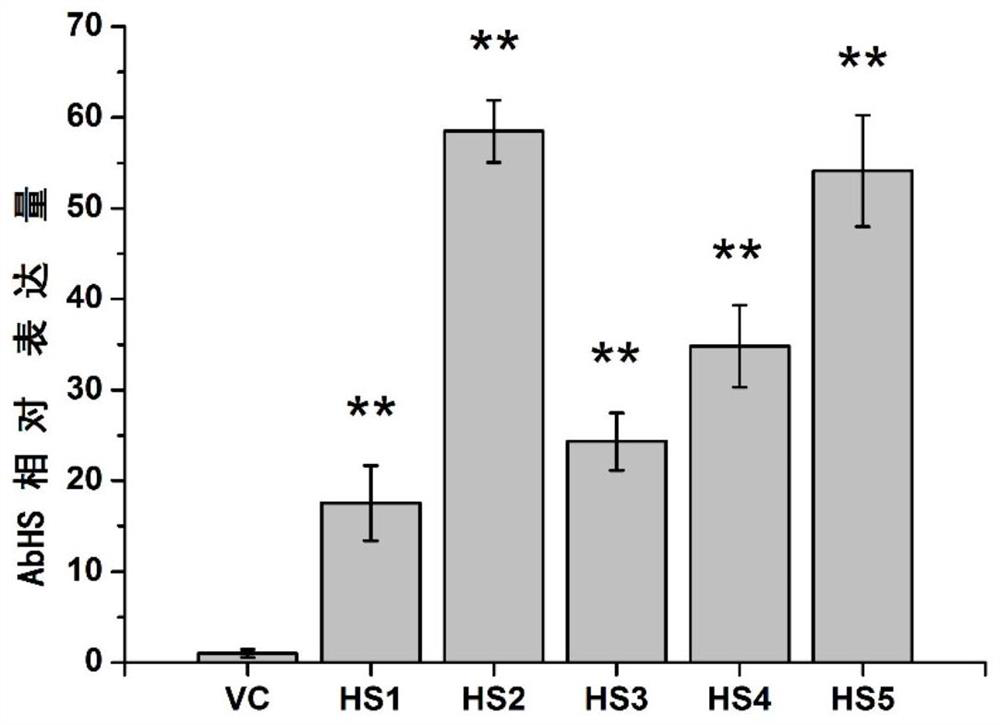
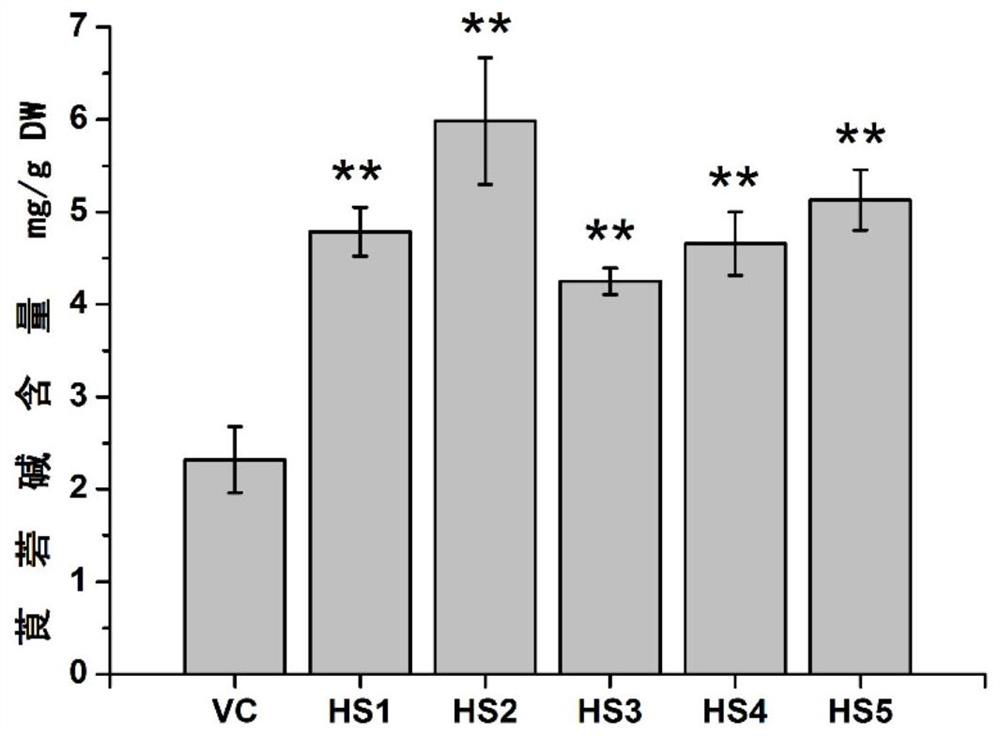
[0054] Example 3, overexpression of HAR increases the content of belladonna hyoscyamine
[0055] (1) Construction of HAR plant overexpression vector
[0056] In order to study the effect of HAR gene on tropine alkaloids in belladonna, the pericycle-specific high expression vector PMT promoter::HAR was constructed, and the original plasmid was pBI121. First of all, the 35S promoter of pBI121 on the original plasmid was replaced with the AbPMT promoter by using the enzyme cutting sites HindIII and XbaI; the AbPMT promoter uses Belladonna cDNA as a template, and the sequences shown in SEQ ID NO.7 and SEQ ID NO.8 are The primers were used for PCR amplification, and then the GUS gene on the original plasmid was replaced by the HAR gene using the restriction sites BamHI and SacI. The HAR gene used the belladonna cDNA as a template, and the sequences shown in SEQ ID NO.9 and SEQ ID NO.10 The primers were used for PCR amplification to obtain the HAR plant overexpression vector PMT pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com