A kind of nickel-based glucose hydrogenation catalyst and preparation method thereof
A hydrogenation catalyst and glucose technology, which is applied in the direction of catalyst activation/preparation, hydroxyl compound preparation, catalyst protection, etc., can solve the problems of flammability, environmental pollution, and poor stability during storage, transportation, and use, and achieve low cost, The effect of shortening the reaction time and stabilizing performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
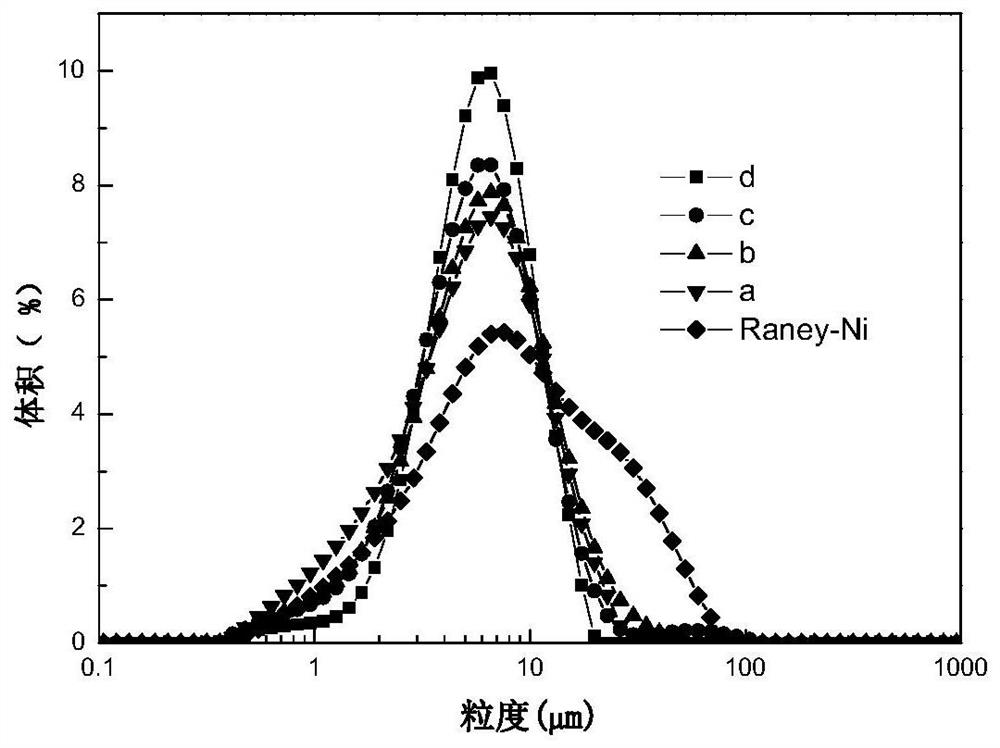
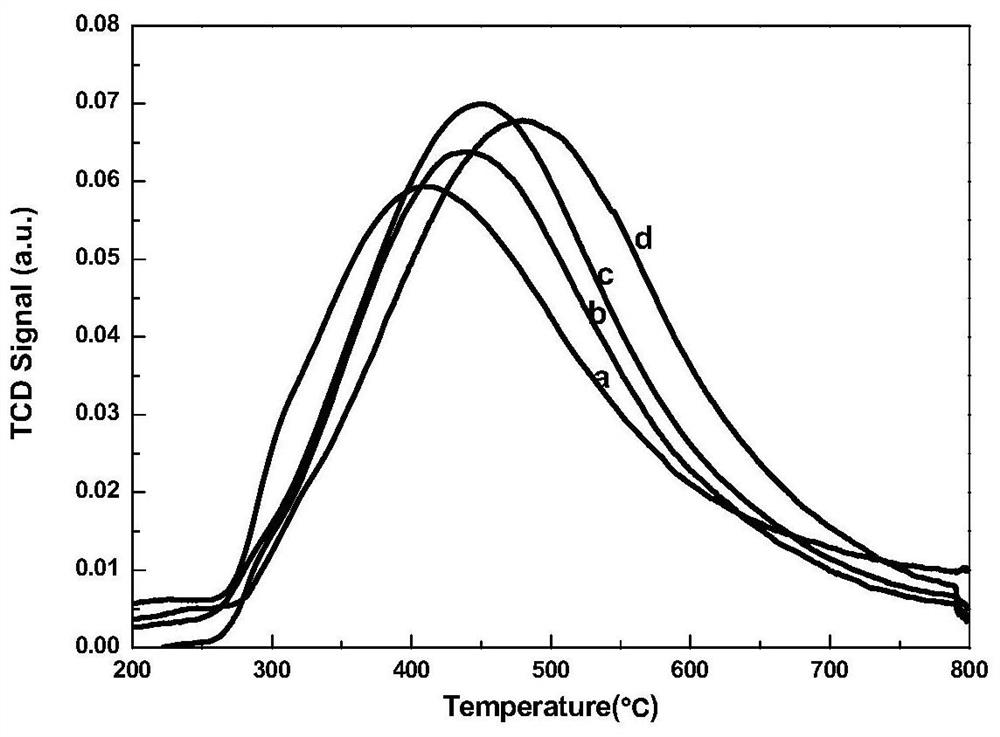
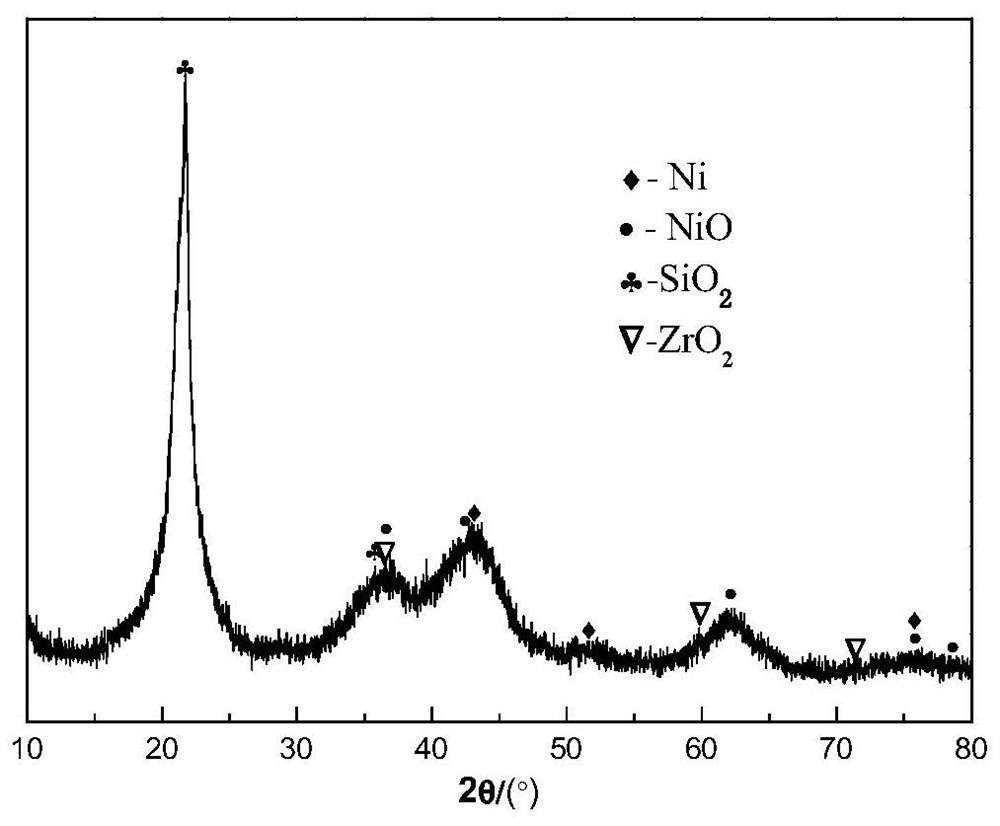
Image
Examples
Embodiment 1
[0028] Example 1 Weigh 25.30g of zirconium nitrate pentahydrate and dissolve it in deionized water to obtain 500mL solution I, weigh 80.85g of sodium metasilicate nonahydrate, 44.28g of sodium carbonate, and add 50.14g of sodium hydroxide into deionized water to obtain 500mL of solution II, weighed 121.0g of nickel nitrate hexahydrate, 24.3g of cobalt nitrate hexahydrate, and 2.0g of polyethylene glycol were dissolved in deionized water to prepare 1000mL of bottom solution. Turn on the stirring, the speed is 650rpm, and the microwave power is 300W, maintain a certain feed rate of solution I and solution II and add it dropwise to the bottom solution, control the pH value of the reaction to 8.0-9.0, and the co-precipitation reaction ends in about 30 minutes. The reaction slurry was aged for 45 minutes. After the aging, the slurry was filtered and washed with water until neutral to obtain a catalyst precursor filter cake.
[0029] Add the filter cake to 2L of deionized water to ...
Embodiment 2
[0033] Embodiment 2 Nickel oxide and nickel are roughly calculated as nickel
[0034]Weigh 25.30g of zirconium nitrate pentahydrate and dissolve it in deionized water to obtain 500mL solution I. Weigh and weigh 80.85g of sodium metasilicate nonahydrate, 37.75g of sodium carbonate, 71.23g of sodium hydroxide, and 34.0g of hexamethylenetetramine. g was added to deionized water to make 1000mL solution II, weighed 241.0g of nickel nitrate hexahydrate, 18.33g of cobalt nitrate hexahydrate, 5.08g of iron nitrate nonahydrate, and 4.0g of polyethylene glycol were dissolved in deionized water to make the bottom solution 1000mL. Turn on the stirring, the rotation speed is 650rpm, and the microwave power is 420W. Maintain a certain feed rate of solution I and solution II and add it dropwise to the bottom solution. The pH value of the reaction is controlled at 8.0-8.5, and the co-precipitation reaction is completed in about 45 minutes. The slurry was reacted for 1 hour. After the aging,...
Embodiment 3
[0039] Weigh 25.30g of zirconium nitrate pentahydrate and dissolve it in deionized water to obtain 500mL of solution I, weigh 50.21g of sodium metasilicate nonahydrate, 71.23g of sodium hydroxide, and add 249.6g of hexamethylenetetramine into deionized water to prepare To obtain 1000 mL of solution II, weigh 241.0 g of nickel nitrate hexahydrate, 18.33 g of cobalt nitrate hexahydrate, 7.62 g of iron nitrate nonahydrate, and dissolve 4.0 g of polyethylene glycol in deionized water to obtain 1000 mL of bottom solution. Turn on the stirring, the rotation speed is 650rpm, and the microwave power is 420W. Maintain a certain feed rate of solution I and solution II and add them dropwise to the bottom solution. The pH value of the reaction is controlled at 8.0-8.5, and the co-precipitation reaction is completed in about 45 minutes. The reaction slurry was aged for 1 hour. After the aging, the slurry was suction filtered and washed until neutral to obtain a catalyst precursor filter ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com