Organic solvent-tolerant lipase mutant and application thereof
An organic solvent and lipase technology, applied in the field of genetic engineering and enzyme engineering, can solve problems such as application limitations and inactivation, and achieve the effects of prolonging life, improving production efficiency, and saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Construction of embodiment 1 mutant expression vector
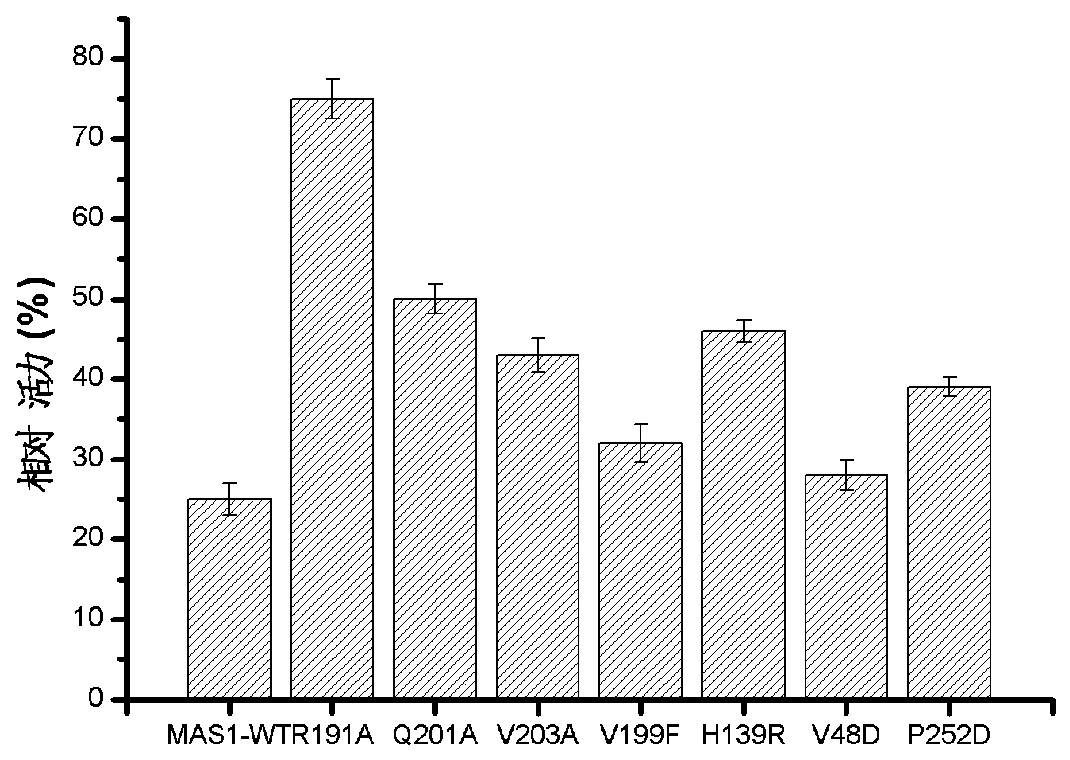
[0026] According to the QuikChange program of Stratagene Company, mutant R191A, Q201A, V203A, V199F, H139R, V48D and P252D primers were designed on the QuikChange primer design website (http: / / www.genomics.agilent.com / primerDesignProgram.jsp). The lipase MAS1 gene was subjected to site-directed mutation in the adopted site-directed mutation (QuikChange Site-directed Mutagenesis). Specifically as shown in Table 1:
[0027] Table 1 Reaction system for site-directed mutation of lipase gene
[0028]
[0029] The PCR reaction conditions were: pre-denaturation at 98°C for 3 minutes; denaturation at 94°C for 10 seconds, extension at 68°C for 4 minutes, 20 cycles; 72°C for 7 minutes. PCR products were checked by 1% agarose gel electrophoresis. After the PCR product was confirmed by detection, DpnI was added to remove the original template strand with methylation. The enzyme digestion system is as follows: enzyme dig...
Embodiment 2
[0031] Embodiment 2 mutant enzyme protein preparation and purification
[0032] The pET22b expression vector containing MAS1 lipase (the amino acid sequence is SEQ ID NO: 1) and its mutant gene is transformed into BL21 (DE3) strains, and induced expression:
[0033] (1) Inoculate 200 μL of positive cloned glycerol bacteria into 50 mL of low-salt LB (containing 100 μg / mL ampicillin) liquid medium, and culture overnight at 37° C. and 200 rpm for 15 to 18 hours.
[0034] (2) Inoculate the seed liquid into 500mL LB (containing 100μg / mL ampicillin) liquid medium according to the inoculum size of 2%, culture at 37°C and 200rpm to OD600 to 0.6-0.8, and then add 1M IPTG solution until the final concentration of IPTG is 0.4 mM, 15°C, 200rpm induction culture for 18h.
[0035] Purification of recombinant protein after induction:
[0036] (1) Bacteria collection: Pre-cool the bacterial solution on ice for about 15 minutes, then centrifuge at 4°C and 10,000 rpm for 10 minutes, and disca...
Embodiment 3
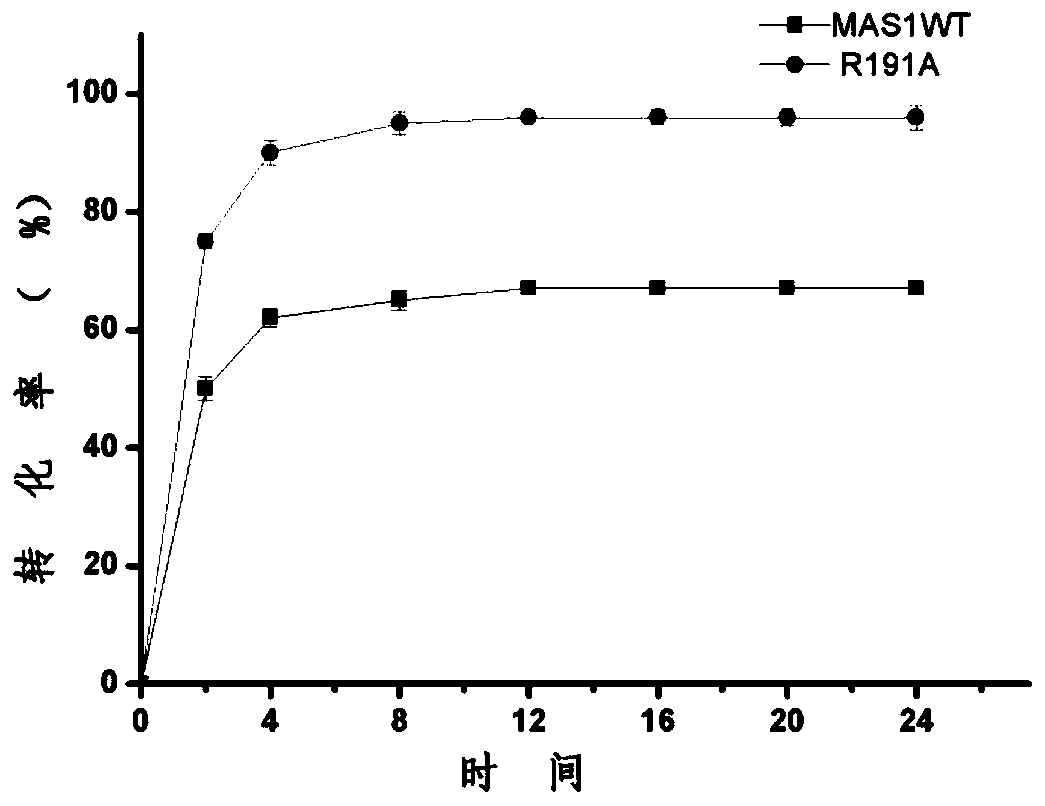
[0039] Example 3 Screening of tolerance to organic solvent mutants
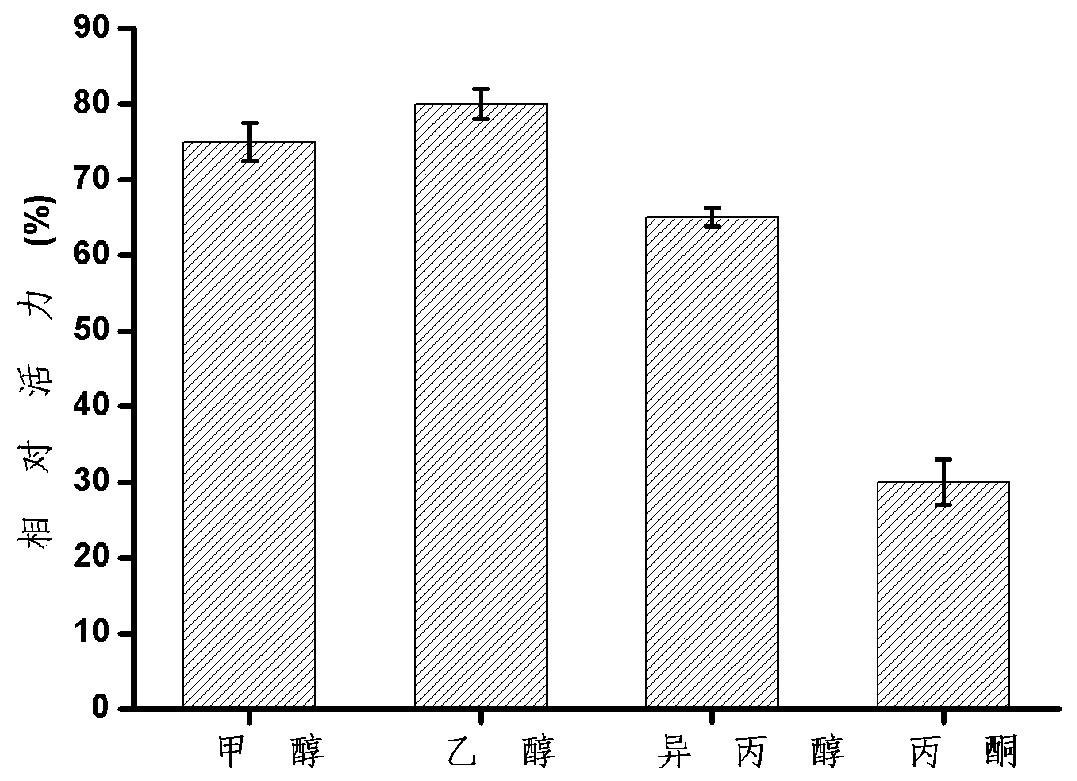
[0040]The enzyme activity was determined by alkaline titration. The specific method was: add 4mL olive oil polyvinyl alcohol emulsion and 5mL buffer solution to a 100mL Erlenmeyer flask with a stopper, preheat for 5min under a constant temperature water bath shaker, and then add 1mL to the experimental group to dilute appropriately. For the control group, 1 mL of the buffer solution in which the protein was added was added, and after 5 minutes of reaction at 200 rpm, 15 mL of 95% ethanol was added to terminate the reaction. After the reaction, add 2 drops of phenolphthalein solution and titrate with 0.05mol / L NaOH standard solution. Under certain reaction conditions, the amount of enzyme required to catalyze the hydrolysis of the substrate to generate 1 μmol of fatty acid per minute is defined as 1 enzyme activity unit, expressed in U.
[0041] The influence of organic solvents on enzyme stability: Add metha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com