Method for determining active proteins in pertussis toxin product and diphtheria-pertussis-tetanus vaccine
A technology for pertussis and diphtheria pertussis, applied in the field of vaccine quality evaluation, which can solve the problems of lack of content determination methods and poor repeatability of vaccine toxins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
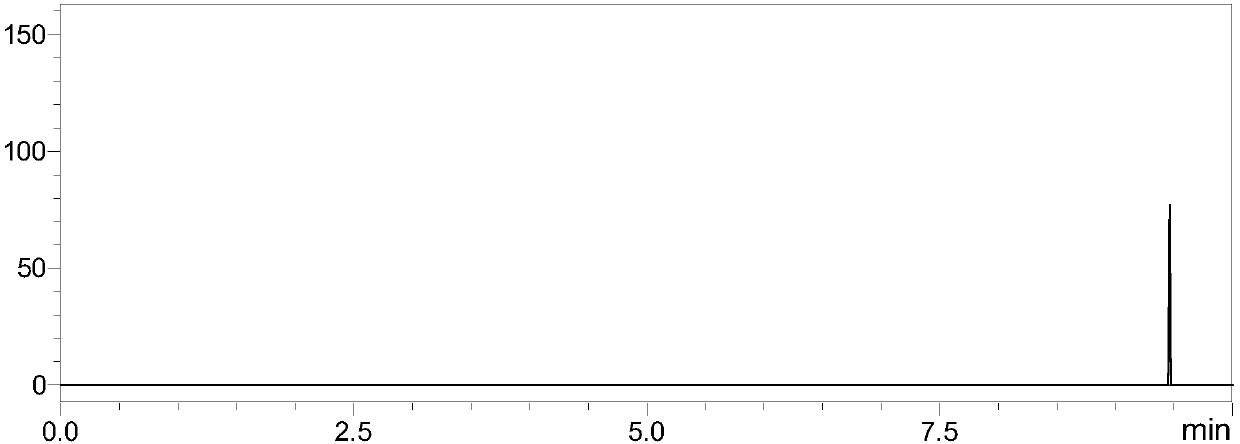
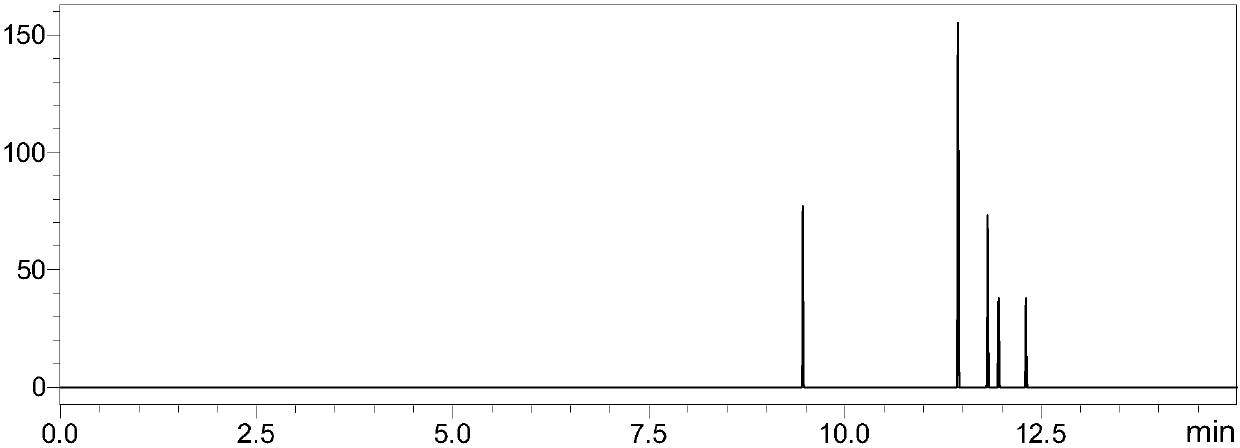
[0118] 1. Experimental instruments and equipment: high-pressure binary pump, degasser, autosampler, column thermostat and triple quadrupole mass spectrometer.
[0119]2. Experimental reagents: Chinese pertussis toxin (PT) standard, filamentous hemagglutinin (FAH, Filamentoushemagglutinin) standard, adhesin (PRN, pertactin) standard, pili protein (FIM, fimbrial) standard, glandular Adenylate cyclase toxin (ACT) standard, dithiothreitol (DTT), iodoacetamide (IAA), ammonium bicarbonate, RapiGest TM , trypsin, formic acid, ultrapure water, and acetonitrile.
[0120] 3. Detection conditions: Chromatographic column: Bi℃18 chromatographic column;
[0121] Mobile phase: A-formic acid: water (1:1000, v / v); B-formic acid: acetonitrile (1:1000, v / v);
[0122] Gradient: 0-8min 5% B-40% B, 8-8.1min 40%-100% B, 8.1-10min 100% B, 10-10.1min 100%-5% B, 10.1-15min 5% B; column temperature : 35°C; flow rate: 0.2-0.5mL / min; injection volume: 10μL.
Embodiment 2
[0156] Example 2: Characterization of EU pertussis toxin standard
[0157] Stationary phase: with stationary phase described in embodiment 1;
[0158] A-formic acid: water (1:1000, v / v); B-formic acid: acetonitrile (1:1000, v / v);
[0159] Gradient: 0-8min 5% B-40% B, 8-8.1min 40%-100% B, 8.1-10min 100% B, 10-10.1min 100%-5% B, 10.1-15min 5% B; column temperature : 35°C; flow rate: 0.2-0.5mL / min; injection volume: 10μL.
[0160] LC-MS conditions: ES+ mode; mass spectrometer: triple quadrupole mass spectrometer; nebulizer flow rate: 3L / min; heater flow rate: 10L / min; interface temperature: 200°C; DL temperature: 235°C; heating module temperature : 400° C.; drying gas flow rate: 10 L / min; interface voltage: 3 kV; the detection mode of the mass spectrometer is multiple ion selective monitoring (MRM), and other detection conditions are the same as in Example 1.
[0161] The enzymatic hydrolysis process of the standard product is as follows: take 100 μL each of the EU pertussis t...
Embodiment example 3
[0163] Implementation Case 3: Characterization of the WHO First Generation Pertussis Toxin (PT) Standard
[0164] Stationary phase: with stationary phase described in embodiment 1;
[0165] A-formic acid: water (1:1000, v / v); B-formic acid: acetonitrile (1:1000, v / v);
[0166] Gradient: 0-8min 5% B-40% B, 8-8.1min 40%-100% B, 8.1-10min 100% B, 10-10.1min 100%-5% B, 10.1-15min 5% B; column temperature : 35°C; flow rate: 0.2-0.5mL / min; injection volume: 10μL.
[0167] LC-MS conditions: ES+ mode; mass spectrometer: triple quadrupole mass spectrometer; nebulizer flow rate: 3L / min; heater flow rate: 10L / min; interface temperature: 200°C; DL temperature: 235°C; heating module temperature : 400° C.; drying gas flow rate: 10 L / min; interface voltage: 3 kV; the detection mode of the mass spectrometer is multiple ion selective monitoring (MRM), and other detection conditions are the same as in Example 1.
[0168] The enzymatic hydrolysis process of the standard product is as follows:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com