Method for efficiently producing silicon tetrafluoride and sodium fluoride by using sodium fluorosilicate
A technology of silicon tetrafluoride and sodium fluorosilicate, which is applied in the fields of materials and chemicals, can solve the problems of silicon tetrafluoride and sodium fluoride fusion agglomeration, long process, complicated operation, etc., and achieve economic and social benefits , reduce production costs, and have strong environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
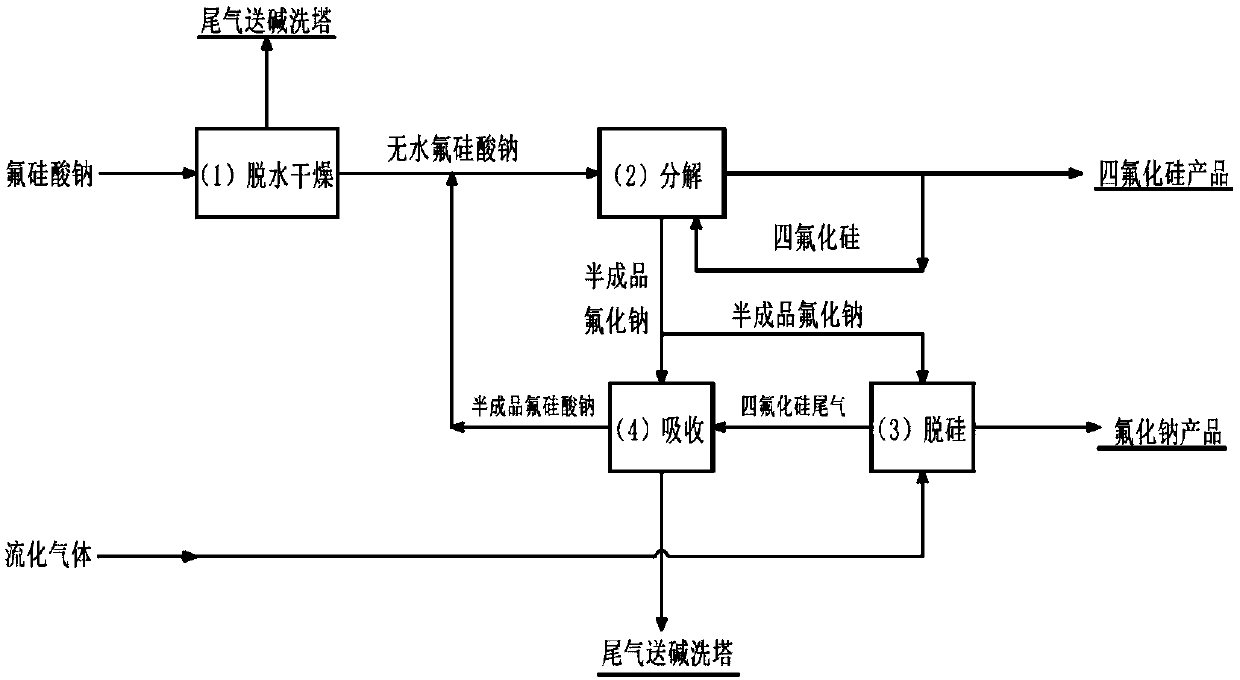
[0028] figure 1 It is a schematic flow chart of the method for efficiently producing silicon tetrafluoride and sodium fluoride by utilizing sodium fluorosilicate described in the present invention.
[0029] combine figure 1 , a method for the efficient production of silicon tetrafluoride and sodium fluoride from sodium fluorosilicate used in this example, including dehydration and drying process 1, decomposition process 2, desiliconization process 3 and absorption process 4 four processes, specifically according to Follow these steps:
[0030] 1) The sodium fluorosilicate raw material is firstly dehydrated and dried to obtain anhydrous sodium fluorosilicate, and the dry tail gas is sent to an alkali washing tower for treatment;
[0031] 2) Anhydrous sodium fluorosilicate is sent to the decomposition process to obtain silicon tetrafluoride gas and semi-finished sodium fluoride, part of the silicon tetrafluoride gas is returned to the decomposition process as fluidizing gas, a...
Embodiment 2
[0035] This embodiment adopts the method for producing silicon tetrafluoride and sodium fluoride as described in Example 1. The dehydration and drying process 1 adopts vacuum drying, the drying and dehydration temperature is 0 ° C, and the drying time is 36 hours; A fluidized bed reactor with a decomposition temperature of 400°C and a residence time of 180 minutes, and the fluidization gas is silicon tetrafluoride gas; the desiliconization process 3 uses a fluidized bed reactor with a reaction temperature of 700°C and a residence time of 15 minutes. The gas is purified air; the absorption step 4 adopts a fluidized bed reactor with a reaction temperature of 25° C. and a residence time of 180 minutes. The fluidization gas is silicon tetrafluoride tail gas produced in the desiliconization step. The impurity silicon content of sodium fluoride product is 0.3wt% (as SiO 2 meter), the recovery rate of silicon in the raw material is 93% (to enter SiF 4 product count).
Embodiment 3
[0037]This embodiment adopts the method for producing silicon tetrafluoride and sodium fluoride as described in Example 1. The dehydration and drying process 1 adopts fluidized bed drying, the drying and dehydration temperature is 200 ° C, and the drying time is 0.1 h; the decomposition process 2 A fluidized bed reactor is used in the process, the decomposition temperature is 700°C, the residence time is 15min, and the fluidization gas is silicon tetrafluoride; the desiliconization process 3 adopts a fluidized bed reactor, the reaction temperature is 400°C, and the residence time is 180min. The fluidization gas is purified nitrogen; the absorption process 4 adopts a fluidized bed reactor with a reaction temperature of 300° C. and a residence time of 15 minutes. The fluidization gas is silicon tetrafluoride tail gas produced in the desiliconization process. The impurity silicon content of sodium fluoride product is 0.35wt% (as SiO 2 meter), the recovery rate of silicon in the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com