Drug carrier with bone targeting as well as preparation method and application thereof
A bone-targeted and targeted technology, applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve the problem of drug transport to the whole body or excessive metabolism, systemic blood drug concentration High, slow drug onset and other issues, to achieve the effect of improving hydrophilicity, increasing yield, and ensuring singleness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] This embodiment provides a drug carrier with bone targeting, including β-cyclodextrin as the main body of the drug, and aspartic acid hexapeptide as the target with bone targeting; the aspartic acid hexapeptide The peptide is linked to β-cyclodextrin via a flexible chain.
[0046] Wherein, the β-cyclodextrin is only connected with one aspartic acid hexapeptide through a flexible chain; the flexible chain is polyethylene glycol.
[0047] The preparation method of the bone targeting drug carrier comprises the following steps:
[0048] (1) Dissolve 1.5 mM modified β-cyclodextrin (β-CD) containing an amino group in PBS buffer, and protect it with argon for 30 minutes;
[0049] (2) Then add 1 mM polyethylene glycol (NHS-mPEG-MAL) solution with maleimide at one end and succinimide at the other end dropwise, and after stirring at room temperature for 1 hour, add 1 mM modified solution with sulfhydryl group at one end dropwise. Aspartic acid hexapeptide (D6), stirred overnigh...
Embodiment 2
[0053] In this example, a quantitative experiment of HA tablets was carried out on the drug carrier (β-CD-mPEG-D6) prepared in the above examples.
[0054] The specific experimental process is as follows:
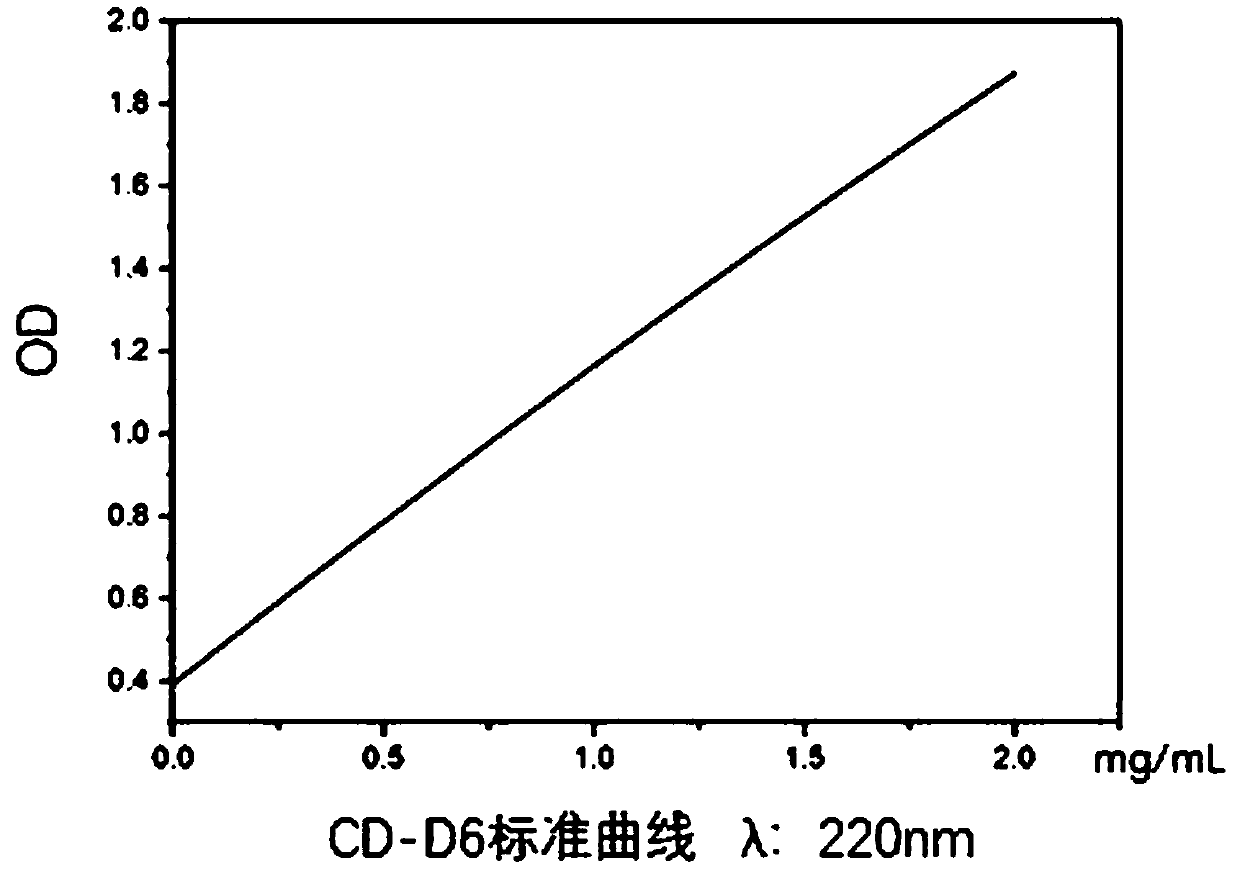
[0055] 1. Make the standard curve of CD-D6: prepare a CD-D6 sample with a concentration of 1mg / mL, and after serially diluting it, use a microplate reader to scan the full spectrum of the CD-D6 sample to obtain the maximum absorption of CD-D6 The wavelength is 220nm; set the wavelength of the microplate reader to 220nm, detect the OD value of the CD-D6 sample after gradient dilution, and make a standard curve of CD-D6, such as figure 1 shown.
[0056] 2. Quantitative adsorption experiment of HA: put the HA disc in the orifice plate, take 1mL CD-D6 sample with a concentration of 1mg / mL on the HA disc with a pipette gun, and place it in a refrigerator at 4°C for 24h, take 100 μL of liquid is used for OD value detection at a wavelength of 220 nm. Using the CD-D6 standard cu...
Embodiment 3
[0059] In this example, an in vitro hydroxyapatite adsorption experiment was performed on the drug carrier (β-CD-mPEG-D6) prepared in the above example.
[0060] Experimental principle:
[0061] In order to make β-cyclodextrin have a fluorescent effect, β-cyclodextrin is used to wrap amantadine labeled with the fluorescent marker FICT. Cavities combine and proportion to match the best substances. Then, FITC was used to label ADA, and then a fluorescence microscope could be used to observe whether the drug carrier interacted with HA.
[0062] The specific experimental process is as follows:
[0063] 1. Use FITC to mark ADA:
[0064] 5mgAd-NH 2 , 1.6mg F-NHS, 1m1 anhydrous DMSO, 50μL anhydrous DIPEA were added to a 1.5ml glass bottle, shake overnight at 25°C, 700rpm, in the dark. The product was separated and purified by high-performance liquid chromatography (HPLC) and then dried with a freeze-concentrated centrifugal dryer;
[0065] 2. Load F-Ada into β-CD and CD-D6 resp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com