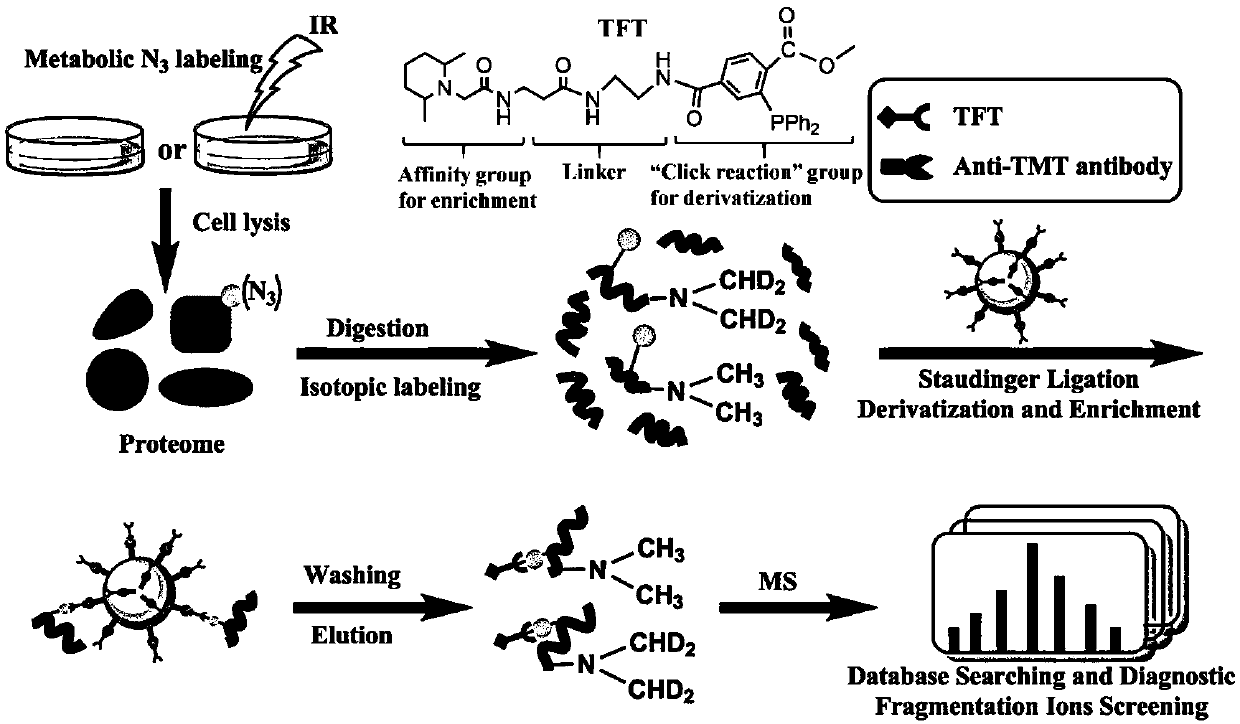
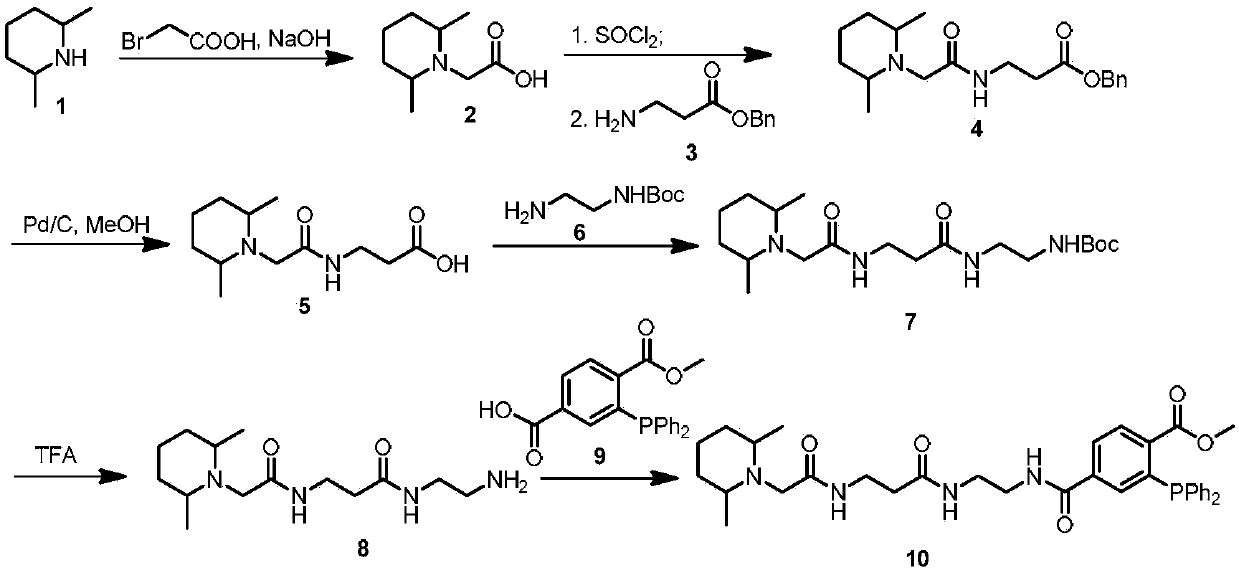
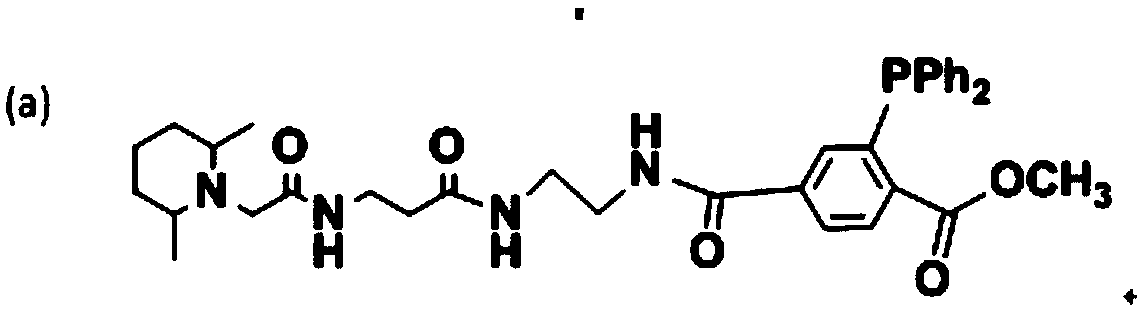
Functional reagent containing trimethylpiperidine group and triphenylphosphine group as well as preparation method and application of functional reagent
A functionalization and phenyl technology, applied in the field of analytical chemistry, can solve the problems of affecting the stability of protein post-translational modification, difficult elution of modified peptides, unfavorable identification, etc., to increase ionization efficiency, improve detection, and increase identification sensitivity and the effect of data size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1, TFT reagent is synthesized as figure 2 As shown, the specific steps are as follows:
[0063] 1.1 Bromoacetic acid substitution reaction to obtain compound 2: Dissolve bromoacetic acid (2.0g, 14.4mmol) in pure water (8mL), add 3.3M NaOH (5mL), adjust the pH to 14, cool to 0°C, and add compound 1 dropwise (1 mL, 11.9 mmol), react in an ice bath for 1 hour, then slowly rise to room temperature for 3 days. The reaction was detected by thin-layer chromatography, extracted three times with dichloromethane, and the product was washed with brine and dried over anhydrous sodium sulfate. It was further purified by silica gel column chromatography (dichloromethane / methanol=20 / 1) to obtain compound 2 (1.3 g, 7.7 mmol, 65%).
[0064] 1.2 Amidation reaction of compound 3 and compound 2 and catalytic reduction reaction of product 4: Dissolve compound 2 (3.0 g, 17.5 mmol) in dry dichloromethane (13 mL), slowly add thionyl chloride dropwise under ice bath (7.77mL, 95.8...
example 3
[0069] Example 3, novel TFT reagent to standard N 3-Evaluation of derivatization efficiency, mass spectrometry detection sensitivity, and MS / MS fragmentation behavior of O-GlcNAc glycopeptides
[0070] Synthetic four criteria N 3 -O-GlcNAc glycopeptides are LNPAVT[N 3 -O-GlcNAc]CAGK (peptide 1), RQLFVT[N 3 -O-GlcNAc]VVK (peptide 2), AQPVQS[N 3 -O-GlcNAc]KPQK (peptide 3) and AAAPAPVS[N 3 -O-GlcNAc]EAVCR (peptide 4), dissolve 10μg glycopeptide (1μg / μL) in PBS, add TFT reagent with a final concentration of 0.5mM, mix evenly with a vortex instrument, seal with a parafilm, and put React in a constant temperature incubator at 37°C for 4 hours. The labeled samples were desalted with a C18 column, freeze-dried, and detected by liquid chromatography-mass spectrometry.
[0071] The labeled peptide was detected by MALDI-TOF-MS, and there was no residual unlabeled standard azide glycopeptide signal in the mass spectrum, indicating that the TFT labeling efficiency reached 100%. Then...
example 4
[0072] Example 4, the antibody resin functionalized by TFT reagents to the N of doped BSA enzyme-cleaved peptides 3 Enrichment efficiency of -O-GlcNAc standard glycopeptides
[0073] In order to evaluate the feasibility of the enrichment method described in the present invention, we first analyzed the N 3 -O-GlcNAc standard glycopeptides were enriched and identified:
[0074] 1) Preparation of TFT functionalized antibody: TFT reagent was added to anti-TMT antibody resin (purchased from thermo fisher scientific company) containing 50 nmol / mL, and incubated at room temperature for 1 h.
[0075] 2) Enrichment: N 3 -O-GlcNAc standard glycopeptides were mixed with BSA enzyme-cleaved peptides at a mass ratio of 1:100, mixed with 25 μL of the TFT functionalized antibody resin obtained in 1), and incubated at room temperature for 4 h. After enrichment, use 100 μl IP buffer containing 0.05% Tween-20 (100 mM HEPES, 250 mM NaCl, 0.2 mM NaCl 2 HPO 4 , pH=7.4) and washed 3 times to re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com