A method for preparing (s)-1,2,3,4-tetrahydroisoquinoline-1-carboxylic acid and derivatives thereof
A technology of substrate and methyl group, applied in the field of biocatalysis, can solve the problem that the optical purity of the product needs to be further improved, and achieve the effects of mild reaction conditions, good selectivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Embodiment 1 Genetic Engineering Bacteria Strain Construction
[0082] 1.1 Screening of D-amino acid oxidase and construction of genetically engineered bacteria expressing D-amino acid oxidase
[0083] According to different substrate specificities, D-amino acid oxidases from microorganisms can be divided into two categories: 1) amino acids (such as D-alanine) with a small side chain group preference for substrates, such as Fusarium oxysporum (Fusarium oxysporum)-derived D-amino acid oxidase; 2) preference for amino acids with larger substrate side chain groups (such as D-phenylalanine), such as Trigonopsis variabilis-derived D-amino acid oxidase ( POLLEGIONI L, MOLLA G, SACCHI S, et al. Properties and applications of microbial D-amino acid oxidases: current state and perspectives [J]. Appl Microbiol Biotechnol, 2008, 78(1): 1-16.). The amino acid sequences of these two D-amino acid oxidases were used for BLASTp analysis in the National Center for Biotechnology Informa...
Embodiment 2
[0126] 2.1 Culture of microorganisms
[0127] Composition of liquid LB medium: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L, dissolved in deionized water and then constant volume, sterilized at 121°C for 20min, ready for use. If it is solid LB medium, add 15g / L agar.
[0128] The engineered bacteria containing the D-amino acid oxidase gene were inoculated in 5 mL of liquid LB (containing 50 μg / ml kanamycin) medium, and cultured with shaking at 200 rpm for about 8 hours at 37°C. Inoculate in 100mL liquid LB (containing 50μg / ml kanamycin) culture medium according to 1% (V / V) inoculum size, OD 600 After reaching 0.6-0.8, add the inducer isopropylthiogalactoside (initial concentration: 0.1 mM), and induce for 15 hours at 18°C. After the cultivation, pour the culture solution into a 100mL centrifuge tube and centrifuge at 4000rpm for 10min, discard the supernatant, collect the bacterial cells, wash the cells twice with 50mM phosphate buffer (pH 8.0), and store them in a -80°C ult...
Embodiment 3
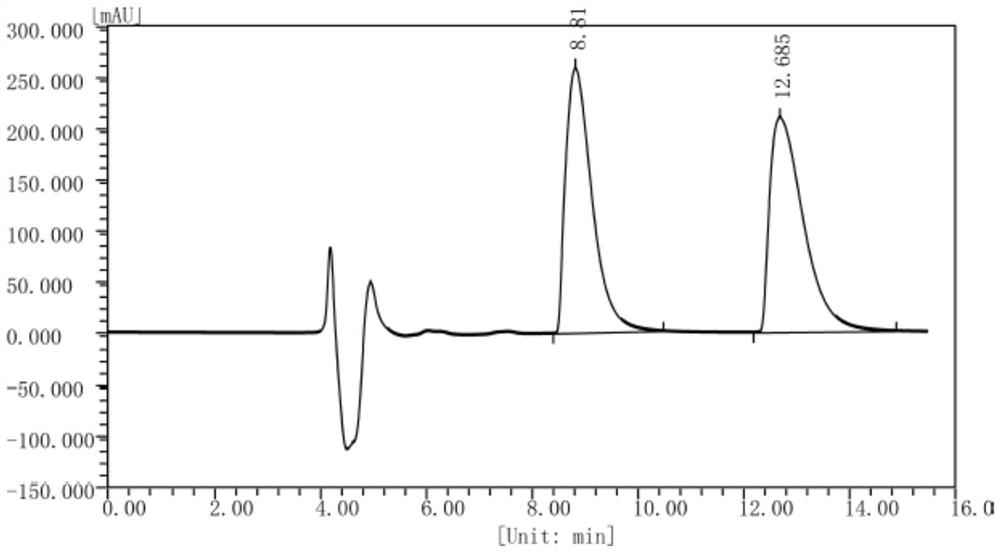
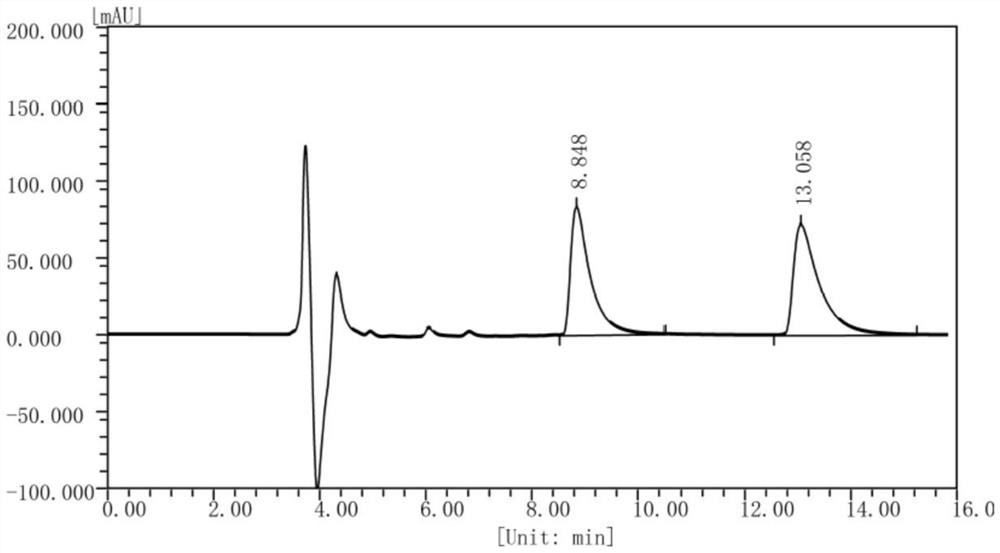
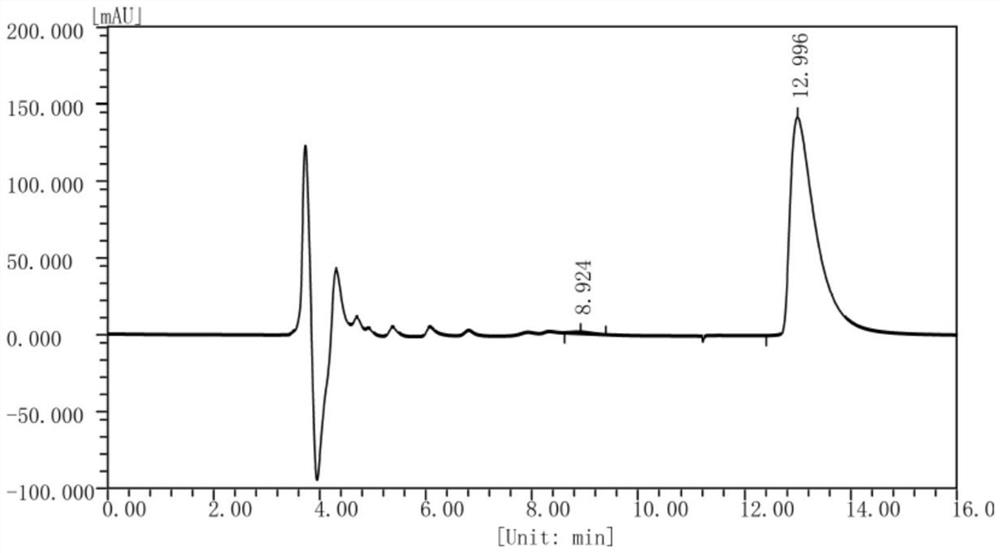
[0131] Example 3 Preparation of (S)-1,2,3,4-tetrahydroisoquinoline-1-carboxylic acid by FsDAAO-PpdpkA multi-enzyme coupling
[0132] According to the method of Example 2, the crude enzyme solution of D-amino acid oxidase derived from Fusarium solani (Fusarium solani) M-0718 and the crude enzyme of pipecolic acid reductase from Pseudomonas putida (Pseudomonas putida) KT2440 were prepared respectively. solution and glucose dehydrogenase crude enzyme solution of Bacillus subtilis (Bacillus subtilis) 168.
[0133] Weigh 0.2g of racemic 1,2,3,4-tetrahydroisoquinoline-1-carboxylate hydrochloride into a 100ml reaction flask, add 10ml of phosphate buffer (50mM, pH=8.0) and mix well, The pH of the solution was adjusted to 8.0 with 30% ammonia water. Add 20ml FsDAAO crude enzyme solution (the crude enzyme solution already contains enough coenzyme FAD, therefore, there is no need to add FAD to the crude enzyme solution reaction system), 7.6ml PpdpkA crude enzyme solution, 2.4ml glucose ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com