Production method of high-purity magnesium sulfite
A technology of magnesium sulfite and production method, applied in magnesium sulfite, magnesium oxide and other directions, can solve the problems of increased desulfurization power consumption, less than 5% market share of magnesium-based desulfurization technology, small crystal particles, etc., to improve the recovery rate , to achieve the effect of value-added resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
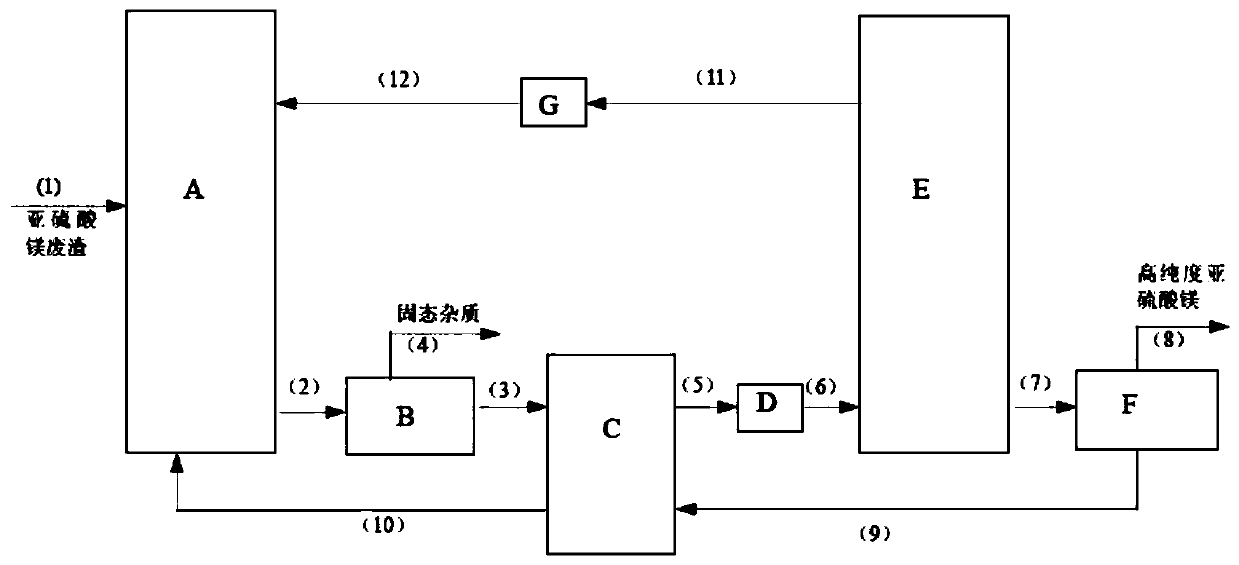
[0033] Embodiment 1: as the description attached figure 1 Shown: Magnesium sulfite waste slag (its components are: 46.7% magnesium sulfite, 39.6% moisture, solid Phase impurity 13.7%.) is added to acidification reactor A by pipeline (1), carries out acidification reaction with the sulfur dioxide gas that introduces acidification reactor A from pipeline (12), and magnesium sulfite crystal dissolves and generates magnesium bisulfite, completes The magnesium bisulfite solution after the acidification reaction enters the impurity removal filter B through the pipeline (2) to remove the solid phase impurities and then enters the heat exchanger C through the pipeline (3), and the solid impurities are discharged through the pipeline (4); In C, the magnesium bisulfite solution and the crystallization mother liquor entering the heat exchanger C from the pipeline (9) are heated to 40°C by countercurrent heat exchange, and then enter the heater D through the pipeline (5) and heat up to 50...
Embodiment 2
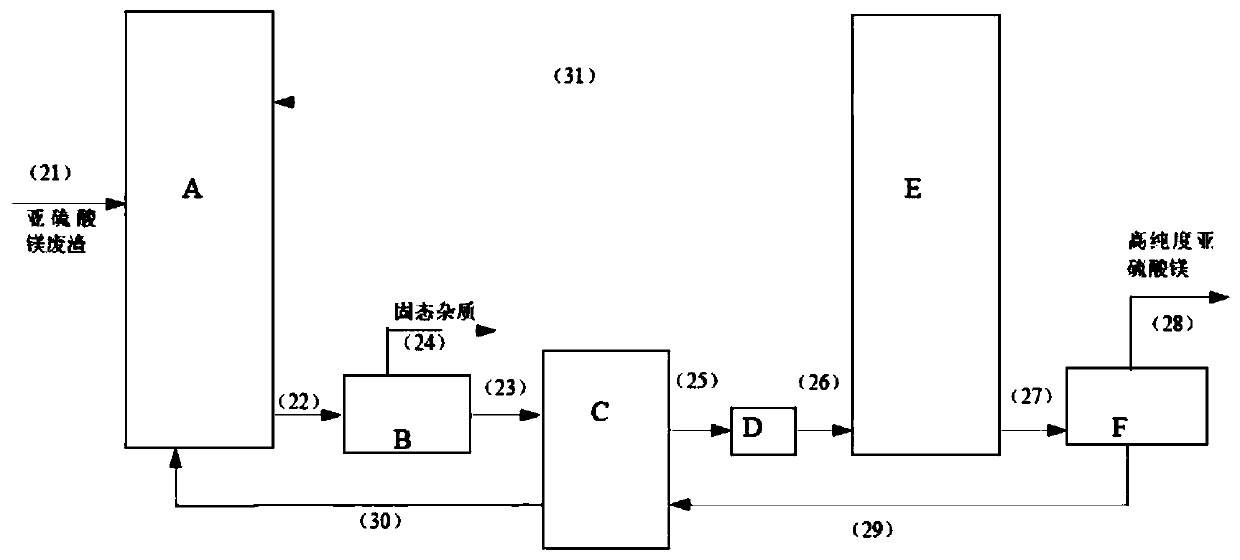
[0036] Embodiment 2: as the description attached figure 2 Shown: Magnesium sulfite waste slag (its components are: 46.7% magnesium sulfite, 39.6% moisture, solid Phase impurity 13.7%.) is fed into acidification reactor A by pipeline (21), carries out acidification reaction with the sulfur dioxide gas that introduces acidification reactor A from pipeline (31), and the magnesium sulfite crystal dissolves and generates magnesium bisulfite, completes The magnesium bisulfite solution of the acidification reaction enters the impurity removal filter B through the pipeline (22) to remove the solid-phase impurities and then enters the heat exchanger C through the pipeline (23), and the solid impurities are discharged through the pipeline (24); Inside, the magnesium bisulfite solution and the crystallization mother liquor that enters the heat exchanger C from the pipeline (29) exchange heat with the countercurrent partition wall and heat up to 80°C, and enter the heater D through the p...
Embodiment 3
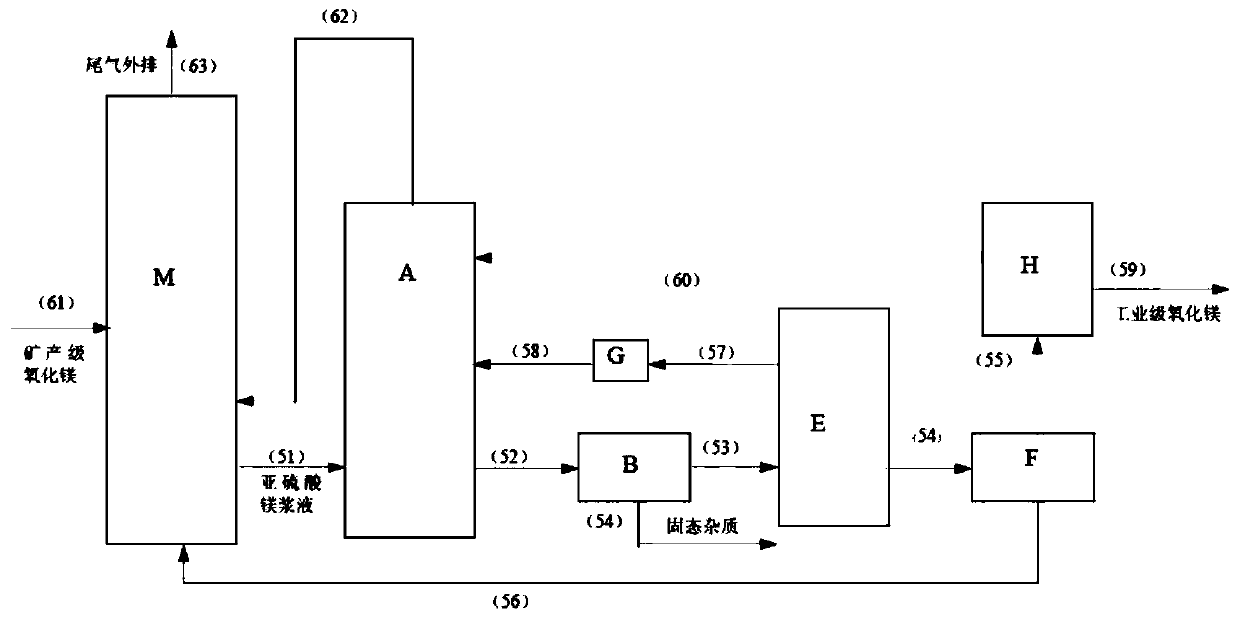
[0039] Embodiment 3: as the description attached image 3 Shown: the magnesium sulfite slurry from the desulfurization absorption tower M is fed into the acidification reactor A through the pipeline (51), and the acidification reaction is carried out with the two streams of sulfur dioxide gas introduced into the acidification reactor A from the pipeline (58) and the pipeline (60) , magnesium sulfite crystals dissolve to generate magnesium bisulfite, and the magnesium bisulfite solution that completes the acidification reaction enters the impurity removal filter B through pipeline (52) to remove solid-phase impurities (the main impurities are calcium sulfite, silicon dioxide and soot) ) into the acidolysis reactor E through the pipeline (53), and the solid impurities are discharged through the pipeline (54); vacuum pump G is used to draw negative pressure in the acidolysis reactor E through the pipeline (57) to maintain the acidolysis reactor E The absolute pressure inside is a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com