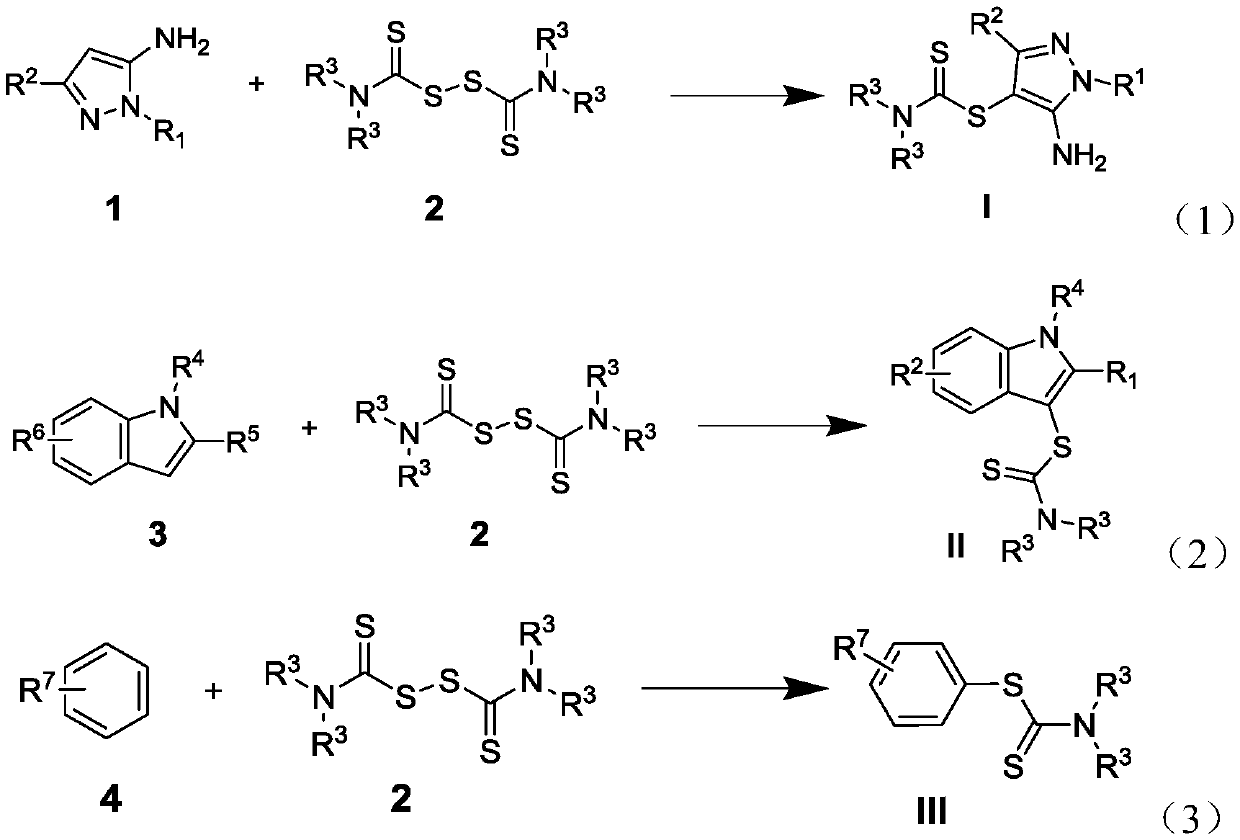
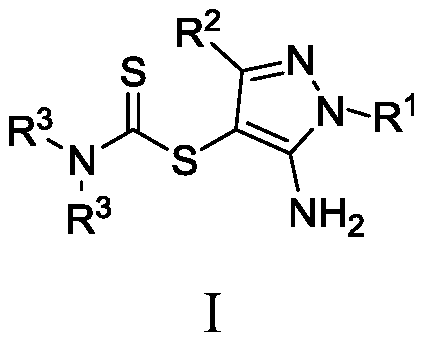
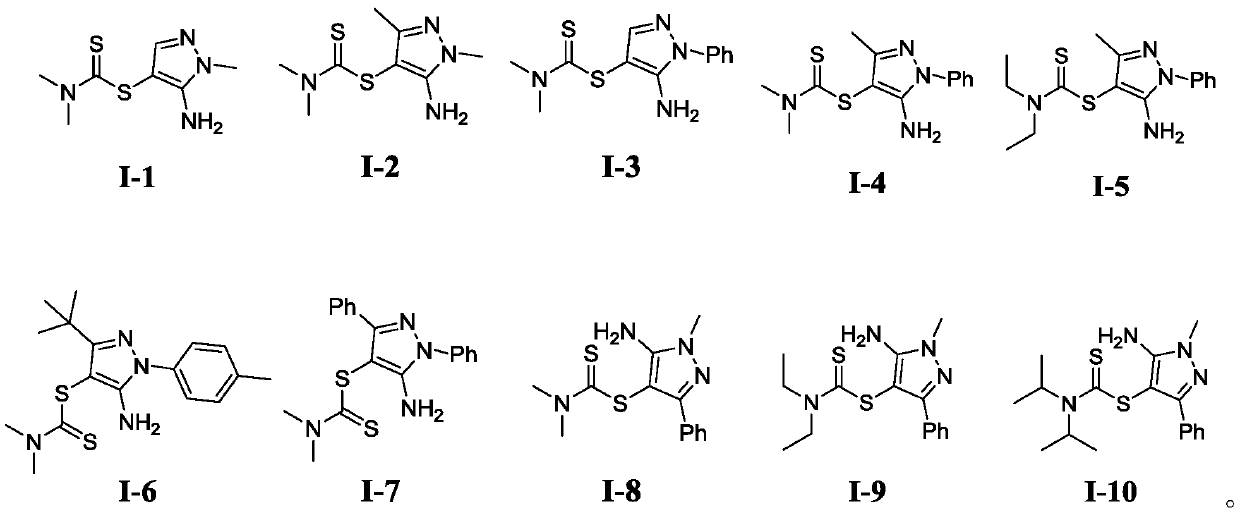
Dithiocarbamate compounds, and preparation method and application thereof
A technology of dithiocarbamate and compounds, applied in the field of chemical biology research, can solve the problems of unsuitable 5-aminopyrazole, interference reaction, high amino activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1. Synthesis of 5-amino-1-methyl-1H-pyrazol-4-yl-dimethylamino-dithiocarbamate (I-1).
[0049] The synthetic route is:
[0050]
[0051] Specifically include the following steps:
[0052] The compound of formula (1a), compound of formula (2a), potassium persulfate, and potassium iodide were mixed in a molar ratio of 1:1.5:2:0.2, wherein the compound of formula (a) was 2 mmol. The reaction system was stirred at 60°C for 12 hours. Cool after the reaction, add an appropriate amount of dichloromethane, extract with water, dry the organic phase, filter with suction, evaporate the filtrate, remove the solvent, and use silica gel column chromatography for the residue, rinse with petroleum ether, detect by TLC, and combine the effluent containing the product , the solvent was distilled off by a rotary evaporator, and the target product was obtained as a white solid by vacuum drying, with a yield of 61% and a purity of 99.5% (HPLC).
[0053] Compound I-1 physical c...
Embodiment 2
[0059] Example 2. Synthesis of 5-amino-1-methyl-3-phenyl-1H-pyrazol-4-yl-dimethylamino-dithiocarbamate (I-8).
[0060] The synthetic route is:
[0061]
[0062] Specifically include the following steps:
[0063] The above formula (1h) compound, formula (2a) compound, potassium persulfate, and potassium iodide were used in a molar ratio of 1:1.5:2:0.2, wherein the formula (1h) compound was 2 mmol. The reaction system was stirred at 60°C for 12 hours. Cool after the reaction, add an appropriate amount of dichloromethane, extract with water, dry the organic phase, filter with suction, evaporate the filtrate, remove the solvent, and use silica gel column chromatography for the residue, rinse with petroleum ether, detect by TLC, and combine the effluent containing the product , the solvent was distilled off by a rotary evaporator, and the target product was obtained as a white solid by vacuum drying, with a yield of 87% and a purity of 99.3% (HPLC).
[0064] Compound I-8 phys...
Embodiment 3
[0070] Example 3. Synthesis of 2-methyl-1H-indol-3-yl-dimethylamino-dithiocarbamate (II-2).
[0071]
[0072] Specifically include the following steps:
[0073] The above formula (3a) compound, formula (2a) compound, potassium persulfate, and potassium iodide were used in a molar ratio of 1:1.5:2:0.2, wherein the formula (3a) compound was 2 mmol. The reaction system was stirred at 60°C for 12 hours. Cool after the reaction, add an appropriate amount of dichloromethane, extract with water, dry the organic phase, filter with suction, evaporate the filtrate, remove the solvent, and use silica gel column chromatography for the residue, rinse with petroleum ether, detect by TLC, and combine the effluent containing the product , the solvent was distilled off by a rotary evaporator, and the target product was obtained as a white solid by vacuum drying, with a yield of 94% and a purity of 99.6% (HPLC).
[0074] Compound II-2 physical constants and characterization data are as fol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com