Preparation method of pH-concentration dependent tertiary amine chromophore polymer
A polymer-dependent technology, applied in the field of responsive fluorescence, can solve the problem of acid-base reversibility and concentration dependence of tertiary amine polymers, no acid-base reversibility and concentration dependence, weak fluorescence intensity of polymers, etc. problems, to achieve the effect of stable autofluorescence performance, simple and stable reaction system, and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
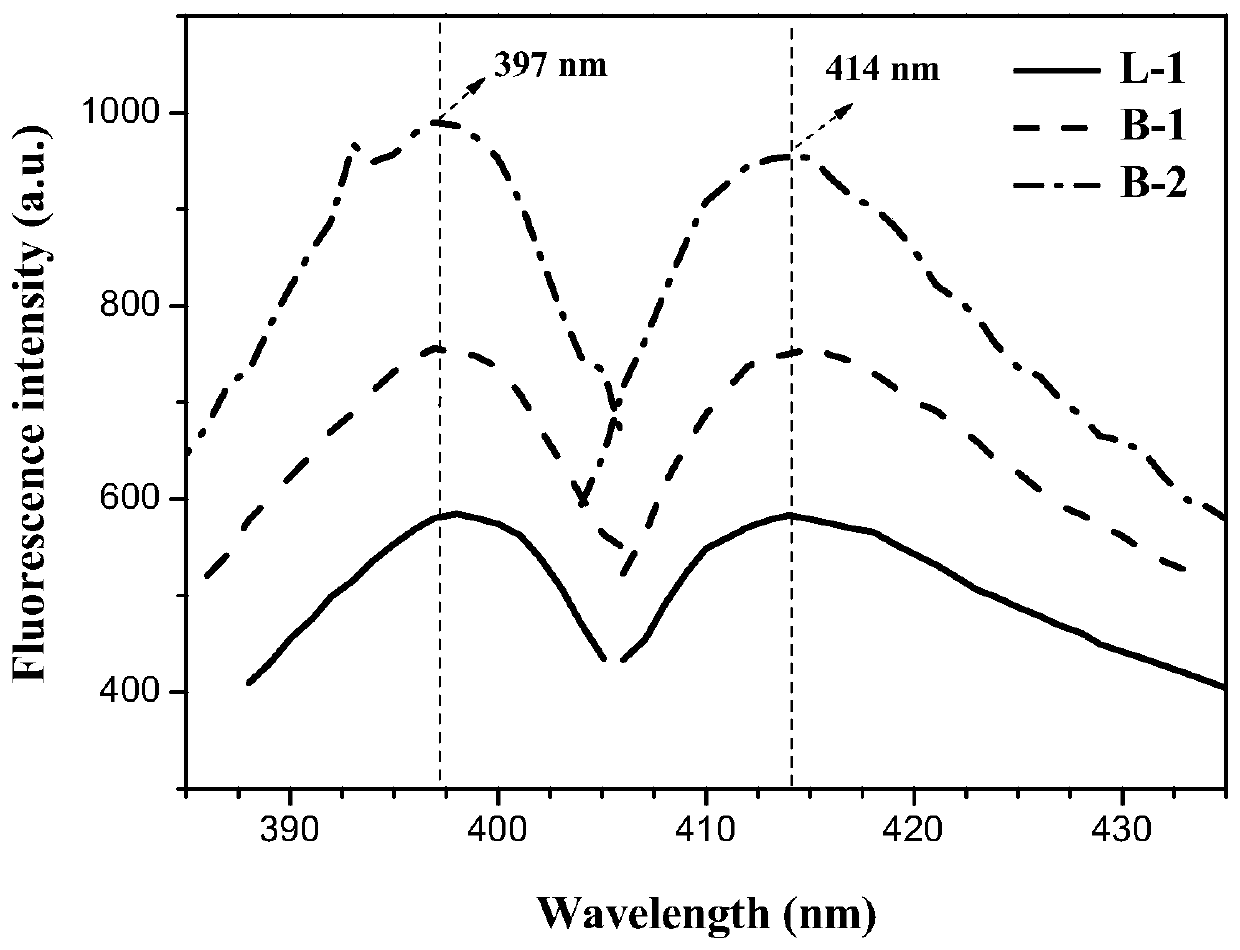
[0037] N-methyldiethanolamine (0.119g, 1mmol), ethylene glycol diacrylate (0.170g, 1mmol) and N,N-dimethylformamide (1.19g, 10mmol) were added to the polymerization bottle, followed by Add the catalyst t-BuP to the polymerization bottle under the protection of argon 2 (50 μL, 0.1 mmol), and reacted at 25° C. for 96 h. After the reaction finishes, add acetic acid to stop the reaction, add methylene chloride to dilute, settle in n-hexane, dialyze through 3500g / mol molecular weight dialysis bag, freeze-dry to obtain the linear polymer (L-1 ), adopt the gel permeation chromatography of DMF phase to characterize the weight-average molecular weight M of polymkeric substance w.GPC =11300g / mol, molecular weight distribution PDI=1.2, the excitation wavelength of the obtained linear tertiary amine polymer in the aqueous solution is 397nm and the emission wavelength is 414nm through the fluorescence spectrum test, and the fluorescence intensity of the polymer presents a gradual increase...
Embodiment 2
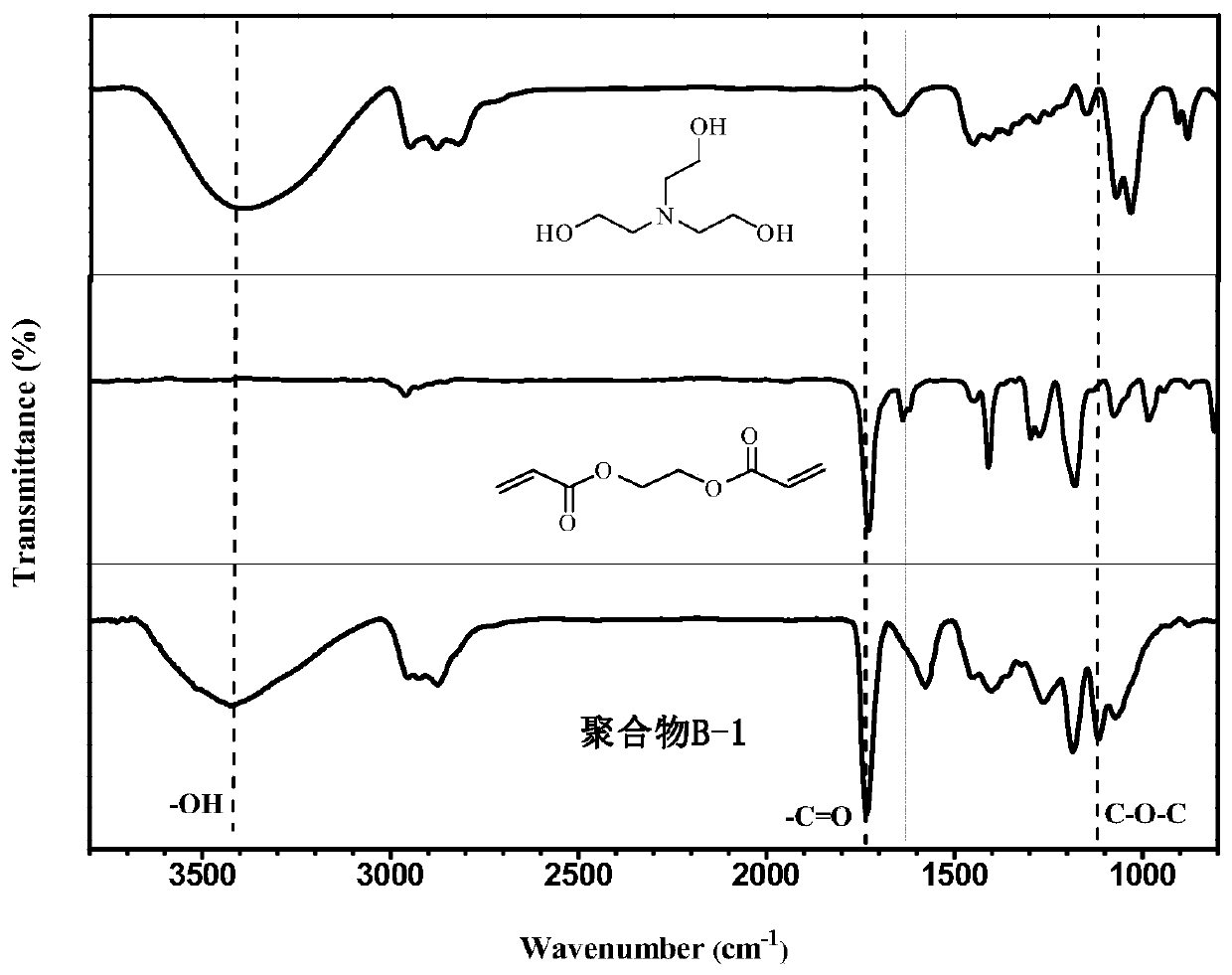
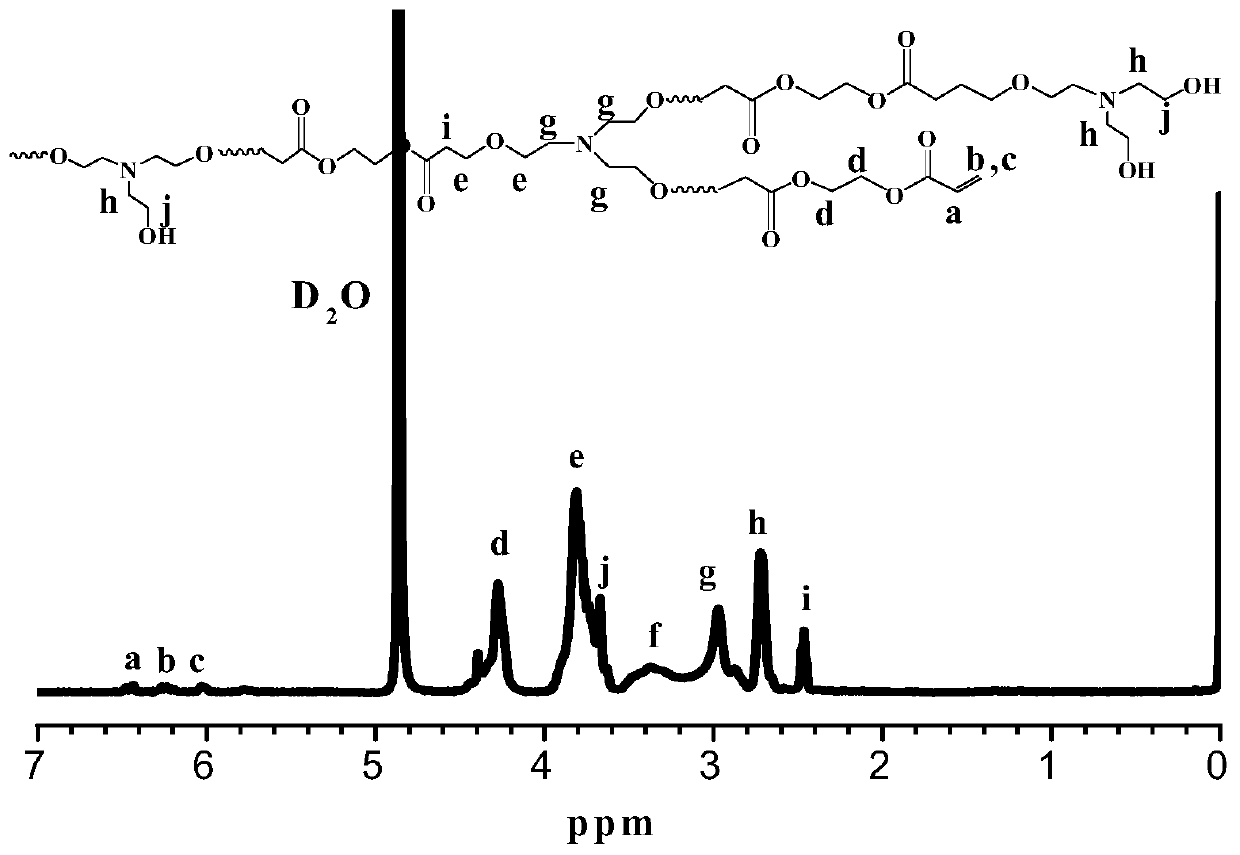
[0039] Triethanolamine (0.149g, 1mmol), ethylene glycol diacrylate (0.170g, 1mmol) and N,N-dimethylformamide (1.19g, 10mmol) were added to the polymerization bottle, followed by Slowly add the catalyst t-BuP into the polymerization bottle2 (50 μL, 0.1 mmol), and reacted at 25° C. for 72 h. After the reaction finishes, add acetic acid to stop the reaction, add dichloromethane and dilute in normal hexane to settle down, obtain the hydroxyl-terminated branched polymer (B -1). The weight-average molecular weight M of the polymer was characterized by gel permeation chromatography using DMF phase w.GPC =19088g / mol, molecular weight distribution PDI=1.68, infrared spectrum and nuclear magnetic spectrum were used to confirm that the polymer with the target structure had been prepared. The excitation wavelength of the obtained hydroxyl-terminated branched tertiary amine polymer in aqueous solution is 397nm, and the emission wavelength is 414nm by fluorescence spectroscopy. The fluore...
Embodiment 3
[0041] Triethanolamine (0.149g, 1mmol), ethylene glycol diacrylate (0.340g, 2mmol) and N,N-dimethylformamide (2.4mL) were added to the polymerization bottle, followed by The catalyst (2.38g, 20mmol) was slowly added into the polymerization bottle, and reacted at 0°C for 72h. After the reaction finishes, add acetic acid to terminate the reaction, add methylene chloride to dilute, settle in normal hexane, dialyze through 3500g / mol molecular weight dialysis bag, freeze-dry to obtain the branched polymer ( B-2). The weight-average molecular weight M of the polymer was characterized by gel permeation chromatography using DMF phase w.GPC =20100g / mol, molecular weight distribution PDI=1.72, infrared spectrum and nuclear magnetic spectrum were used to confirm that the polymer with the target structure had been prepared. The obtained double bond-terminated branched tertiary amine polymer has an excitation wavelength of 397nm and an emission wavelength of 414nm in a 2 mg / mL aqueous so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com