Naphthalimide benzimidazole compound, preparation method and application thereof
A technology of naphthalimide and benzimidazole, which is applied in the field of medicinal chemistry, can solve the problems of undiscovered antimicrobial activity and excellent compounds, and achieves the effects of strong in vitro antimicrobial activity, simple structure and resistance to drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
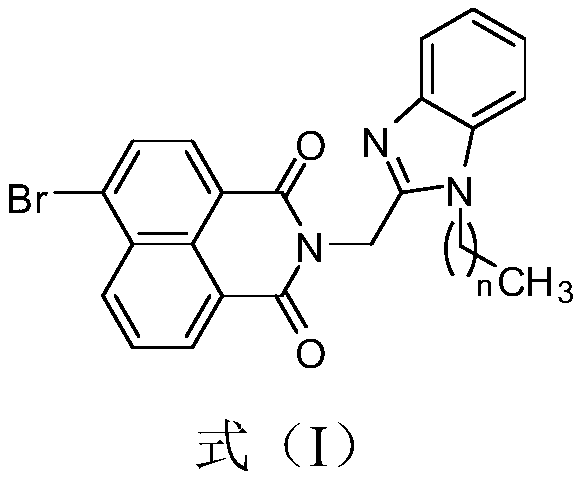
[0029] Preparation of naphthalimide benzimidazole compound I-1, the reaction formula is as follows:
[0030]
[0031] Specific steps are as follows:
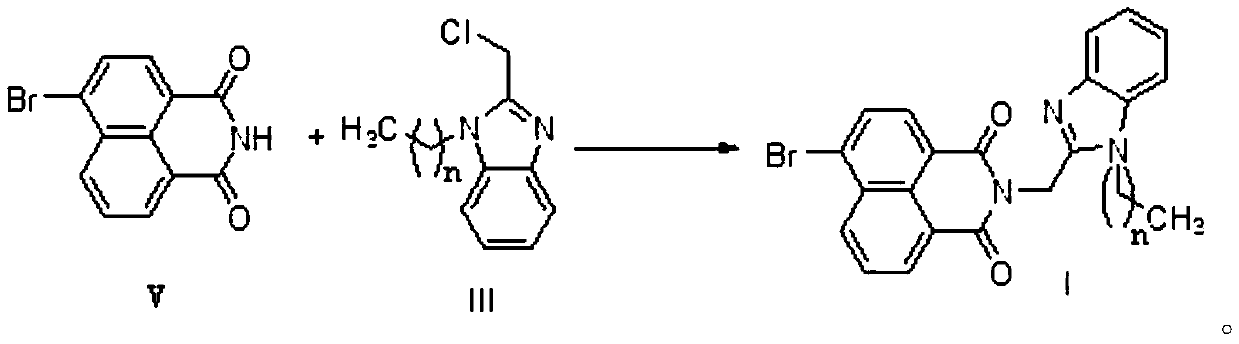
[0032] Weigh 1.195g of 4-bromo-1,8-naphthalimide, 10.912g of 2-(chloromethyl)-1-propyl-1H-benzimidazole III-10.912g and 0.768g of potassium carbonate in a 100mL round bottom flask , add a small amount of DMF to dissolve the system, put the round bottom flask into an oil bath, install a reflux device, heat and stir the reaction at 60°C, track with thin layer chromatography until the end of the reaction, cool to room temperature, and remove the organic solvent DMF by distillation under reduced pressure The residue was obtained, and the residue was separated and purified by silica gel column chromatography, using a mixture of dichloromethane and acetone with a volume ratio of 20:1 as the eluent, and the separated product was dried to obtain the brown syrupy naphthoyl Imine benzimidazole compound I-1.
[0033] The present embod...
Embodiment 2
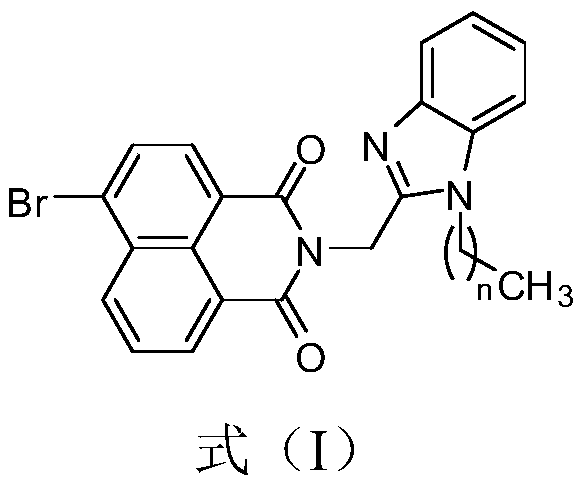
[0036] Naphthalimide benzimidazole compound I-2, the reaction formula is as follows:
[0037]
[0038] Specific steps are as follows:
[0039] Weigh 1.204g of 4-bromo-1,8-naphthalimide, 2-(chloromethyl)-1-hexyl-1H-benzimidazole III-21.097g and 0.837g of potassium carbonate in a 100mL round bottom flask, Add a small amount of DMF to dissolve the system, put the round-bottomed flask into an oil bath, install a reflux device, heat and stir the reaction at 60°C, follow up with thin-layer chromatography until the end of the reaction, cool to room temperature, and distill off the organic solvent DMF under reduced pressure to obtain The residue is separated and purified by silica gel column chromatography, using a mixture of dichloromethane and acetone at a volume ratio of 20:1 as the eluent, and the separated product is dried to obtain the brown syrupy naphthalene Ambenzimidazole compound I-2.
[0040] The present embodiment yield 51%; 1 H NMR (400MHz, DMSO-d 6 ):δ0.73(t,3H,J...
Embodiment 3
[0046] Adopt the 96-hole microdilution method of the clinical experiment standard (National Committee for Clinical Laboratory Standards, NCCLS) that meets the American National Committee formulate in 1993, the naphthalimide benzimidazole compound that embodiment 1-2, comparative example 1 make In vitro antimicrobial activity test, testing these compounds against Staphylococcus aureus, MASR, Micrococcus luteus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Proteus, Candida utilis, Aspergillus flavus, Saccharomyces cerevisiae The minimum inhibitory concentration (MIC) for Candida albicans and Candida.
[0047] The specific test method is: dissolve the compound to be tested with a small amount of dimethyl sulfoxide, then dilute it with water to make a solution with a concentration of 1.28 mg / mL, then dilute it to 1024 μg / mL with culture medium, and incubate at 35°C for 24-72 hours. After the culture plate was placed on a shaker and fully stirred, the MIC value was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com