Potato X virus resistant attenuated vaccine as well as preparation method and application thereof
An attenuated vaccine and potato technology, which is applied in the field of plant anti-virus genetic engineering, can solve the problems of mutant virulent strains, few types of attenuated strains, and inconvenient application, so as to reduce damage, delay plant disease, and stabilize the effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
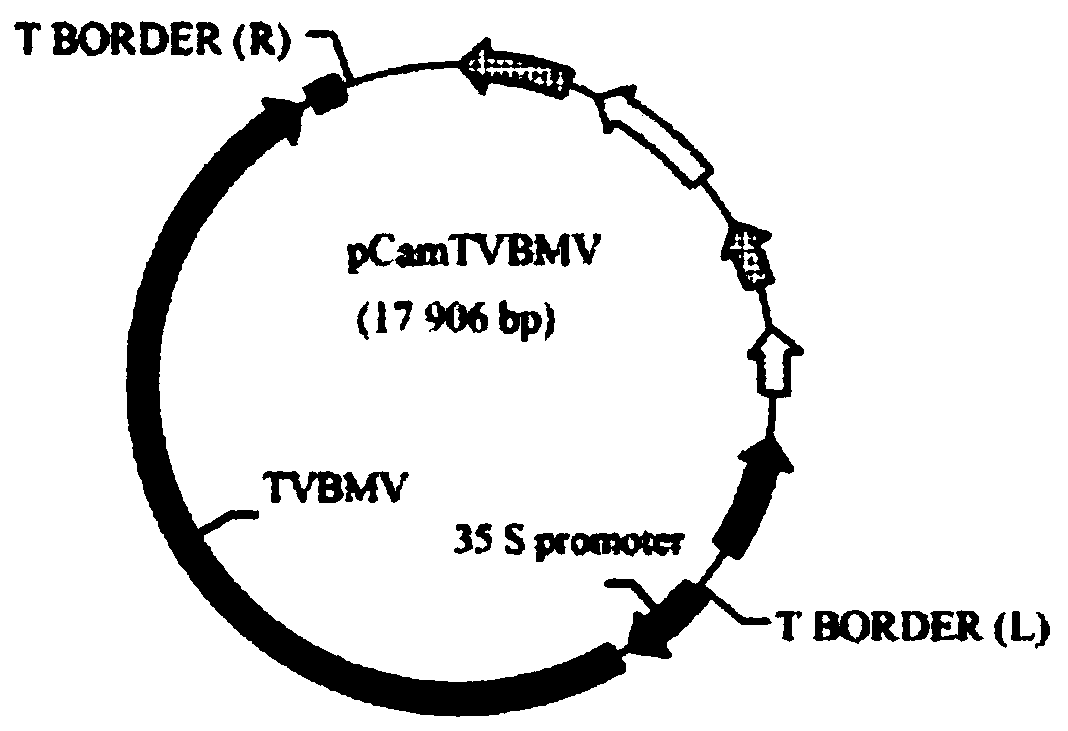
[0043] Example 1 Construction of multi-site TVBMV attenuated mutants
[0044] 1. Construction of Infectious Clones of Tobacco Vein Mosaic Virus
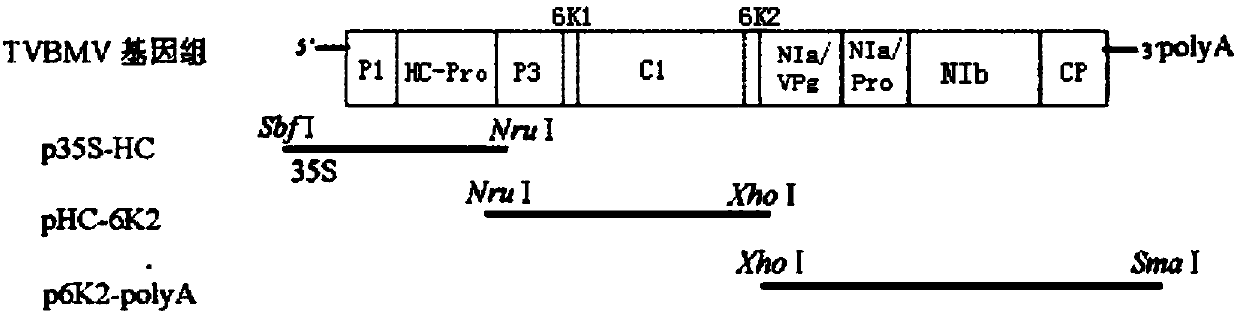
[0045]Using the RNA of tobacco vein mosaic virus as a template, reverse transcription was performed with random primers. According to the restriction enzyme digestion map of the existing tobacco vein mosaic virus genome, it can be amplified in three parts, and assembled into a TVBMV full-length cDNA clone after enzyme digestion. First, the 35S promoter was fused to the upstream of the fragment from the 5′ untranslated region of TVBMV to the Nru I restriction site of the HC-pro gene by Overlap-PCR, and the inventor named the fragment p35S-HC; PCR amplification of HC- The fragment between the Nru I restriction site of the pro gene and the 6K2 Xho I restriction site was named pHC-6K2 by the inventor; the 6K2Xho I restriction site was amplified by PCR to the tail of the poly(A) A fragment, which the inventors named p6K2-polyA (as figu...
Embodiment 2
[0063] Example 2 Amplification of Potato X Virus Related Gene Fragments and Construction of Attenuated Vaccine
[0064] 1. Amplification of potato X virus-related gene fragments
[0065] Using the PVX-1985 genome as a template, RT-PCR was used to amplify each gene fragment. The embodiments provided by the present invention are all in accordance with conventional experimental conditions, and the primer sequences used are as follows:
[0066] Table 2 PVX gene fragment amplification primer sequences
[0067]
[0068]
[0069] Among them, primers 1 and 2 are used to amplify the Rd1 region of PVX, primers 3 and 4 are used to amplify the Rd2 region of PVX, primers 5 and 6 are used to amplify the Rd3 region of PVX, and primers 7 and 8 are used to amplify the PVX region. The CP region, primers 9 and 10 were used to amplify the TGB region of PVX. The nucleotide sequence of the amplified Rd1 gene is shown in Seq ID No.17, the nucleotide sequence of the Rd2 gene is shown in Seq ...
Embodiment 3
[0083] Example 3: Chimeric virus inoculated plants
[0084] The chimeric virus obtained in Example 2 was transformed into Agrobacterium GV3101. After verification by colony PCR, recombinant bacteria were obtained. Then single spot was inoculated in liquid LB medium containing kanamycin (50 μg / mL), rifamycin (50 μg / mL) and tetracycline (50 μg / mL). Add 500 μL of bacterial liquid to 5 mL of LB medium containing 10 mmol / L 2-(N-morpholine)-ethylsulfonic acid (MES), 20 μmol / L acetosyringone (AS) and the above three antibiotics, at 28°C Shake culture to logarithmic growth phase.
[0085] Cells were collected by centrifugation and resuspended in 10 mmol / L MgCl 2 , 10mmol / LMES, 150μmol / L AS, adjust the concentration to make the OD 600 is about 0.5, and let stand for 3 hours at room temperature. Take a 5mL disposable syringe, remove the needle and absorb the Agrobacterium solution, and infiltrate the back of the leaves of common tobacco (5-6 weeks old or 4-6 true leaves). Infiltra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com