A method for preparing decanediol dicarboxylate and derivatives thereof
A technology of decanediol dicarboxylate and carboxylic acid, which is applied in the field of chemical preparation and can solve problems such as limited sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] NHC catalyst cycle:
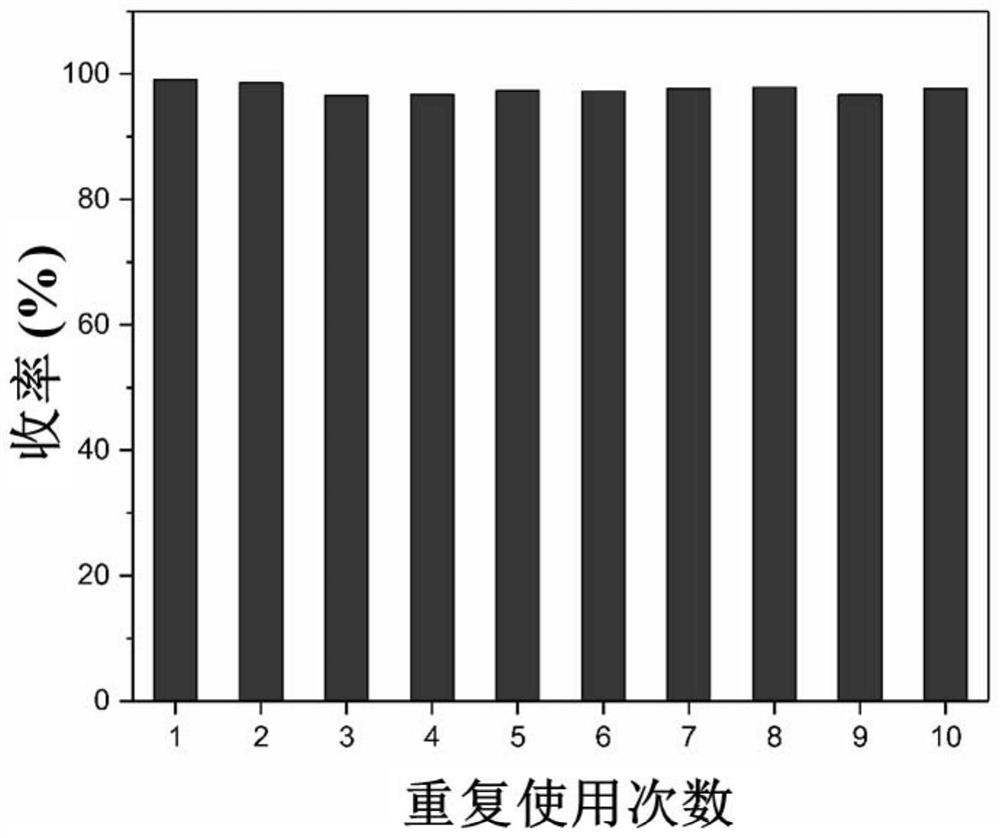
[0063] Under an inert atmosphere, sequentially add 0.5mmoLNHC catalyst into a 100mL Shrek tube And 2mmoL triethylamine, then add 0.1moL furfural, react at 80°C for 3h, after the reaction, the product is dissolved in acetic acid and filtered. The obtained filter residue was successively washed three times with methanol and three times with acetone. After drying, put it into a Shrek bottle, then add 2mmoL triethylamine and 0.1moL furfural, operate according to the above process, repeat 10 times, and measure the yield of furoin by HPLC as follows: figure 1 shown.
Embodiment 2
[0065] Under an inert atmosphere, add 0.005mmoL NHC catalyst to the Shrek tube successively Mix 0.02mmoL triethylamine, then add 1.0mmoL furfural, and react at 80°C for 3h to obtain furoin. Dissolve 0.5moL of the obtained furoin with acetic acid, filter to remove the NHC catalyst, transfer the filtrate to a high-pressure reactor, and then add 0.05mmoL Sc(OTf) 3 and 0.04mmoL Pd / C, filled with 4MPa H 2 , reacted at 185°C for 60min. After the mixed product is filtered and vacuum distilled, decanediol diacetate and n-decyl acetate can be obtained respectively.
[0066]
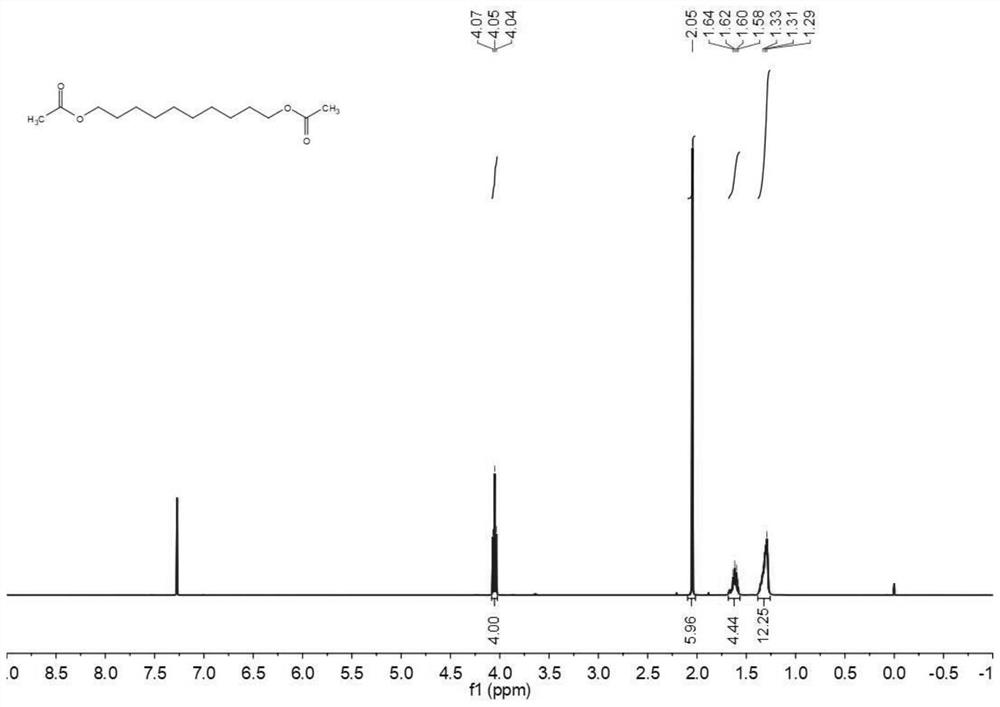
[0067] The H NMR spectrum of decanediol diacetate figure 2 Shown: 1 H NMR (400MHz, CDCl 3 ): δ=4.00(t, 6.00Hz, 4H), 2.05(s, 6H), 1.68-1.63(m, 6H), 1.40-1.25(m, 12H).
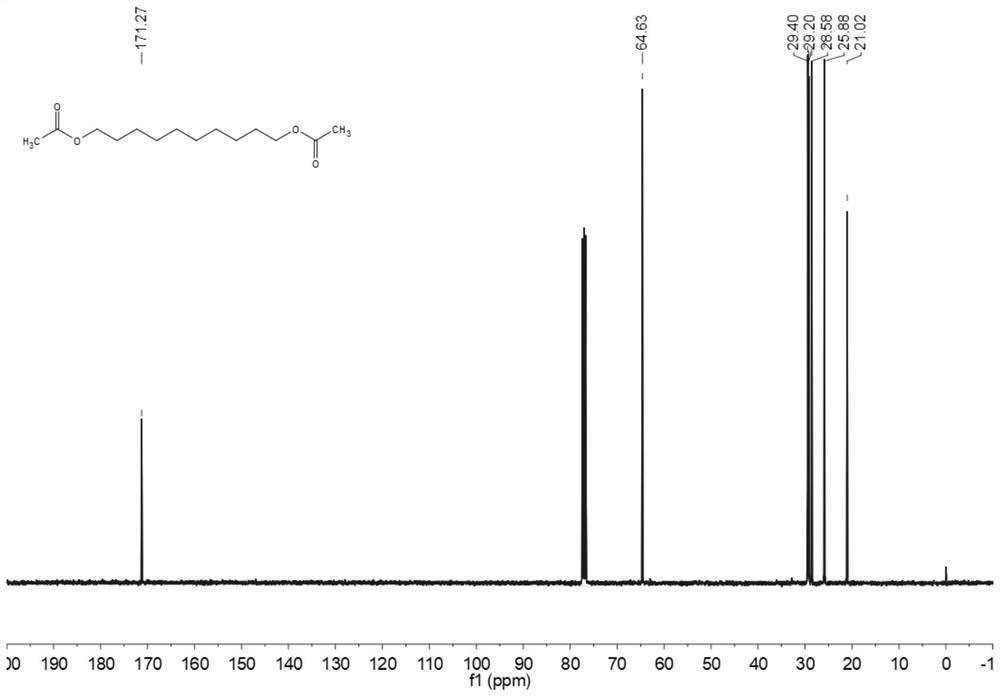
[0068] The carbon NMR spectrum of decanediol diacetate is as image 3 Shown: 13 C NMR (101MHz, CDCl 3 ): δ = 171.27, 64.63, 29.40, 29.20, 28.58, 25.88, 21.02.
[0069] The H NMR spectrum of n-decyl acetate is as Figure 4 Shown: 1 H...
Embodiment 3
[0074] Take 5.0g furfural and 100mL THF into a 500mL four-neck round bottom flask, stir at -10°C for 10min, then within 30min, add 11.83g TiCl 4 Add dropwise to the flask. After another 30 min, 8.1 g of Zn powder was divided into small portions and added to the flask within 30 min. After the addition was complete, the mixture was kept stirring at -10 °C for 1 h, then warmed to room temperature and refluxed overnight. After the reaction was completed, the reaction was quenched with ice water and filtered. The resulting solid was dissolved in dichloromethane and filtered. The obtained filtrate was extracted 2-3 times with dichloromethane, and the dichloromethane was removed by rotary evaporation. The resulting crude product was purified by silica gel chromatography to give a white solid, namely 1,2-difurylethene. Take 0.5mmoL of the 1,2-difurylethylene obtained in the above steps and place it in the autoclave, then add 0.05mmoL of Sc(OTf) 3 , 0.04mmoL Pd / C and 10mL acetic a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com