A kind of multi-functional triaryl amino polyamide containing fused ring naphthyl side group structure and its preparation method and application
A triarylamine-based polyamide, multi-functional technology, applied in the preparation of amino compounds, organic compounds, amino compounds from amines, etc., can solve the problems of single function, poor heat resistance, poor solubility, etc. The yoke area, improve the bonding strength, beneficial to the effect of the test
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
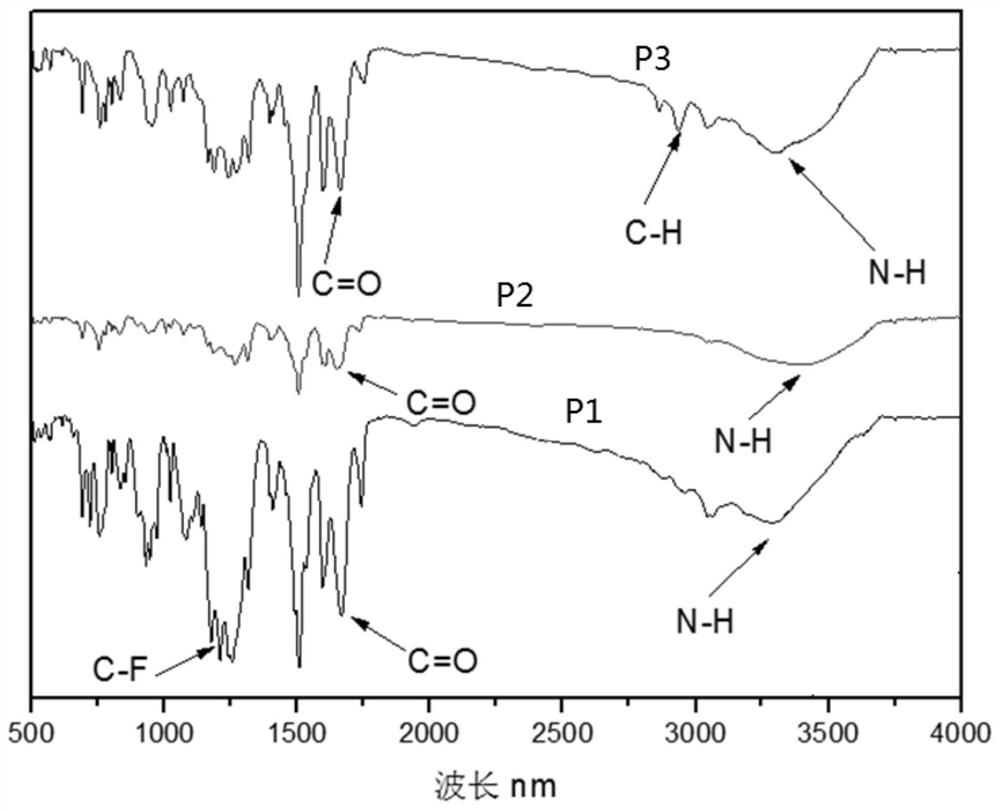
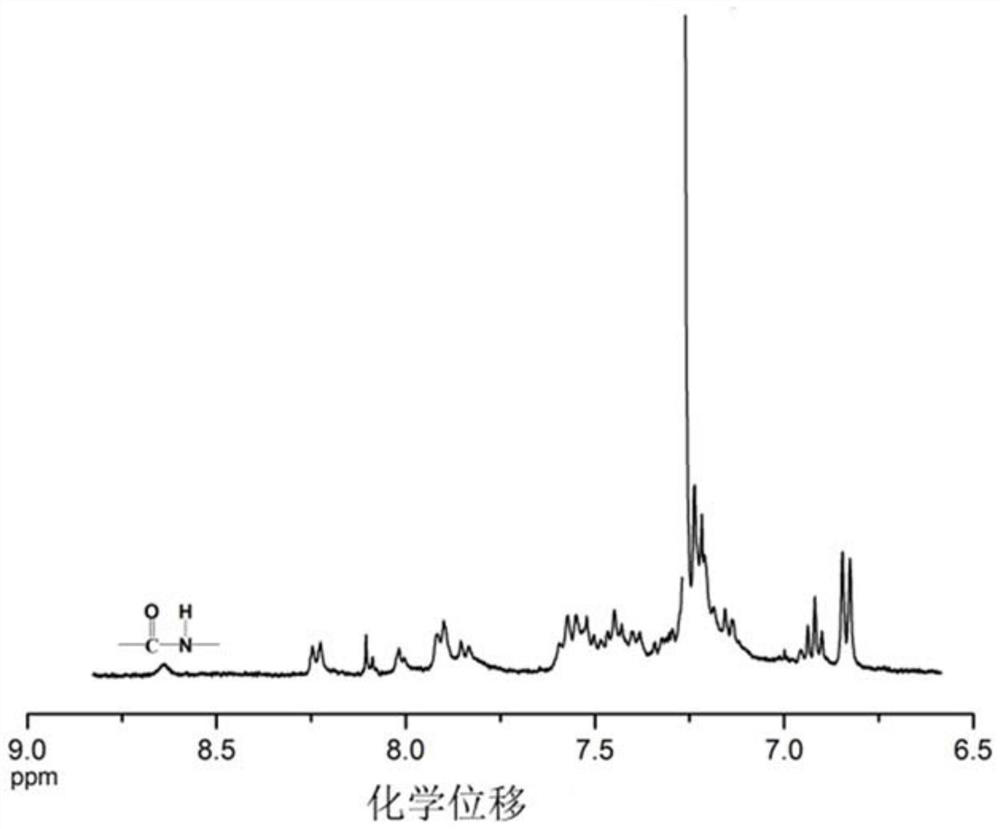
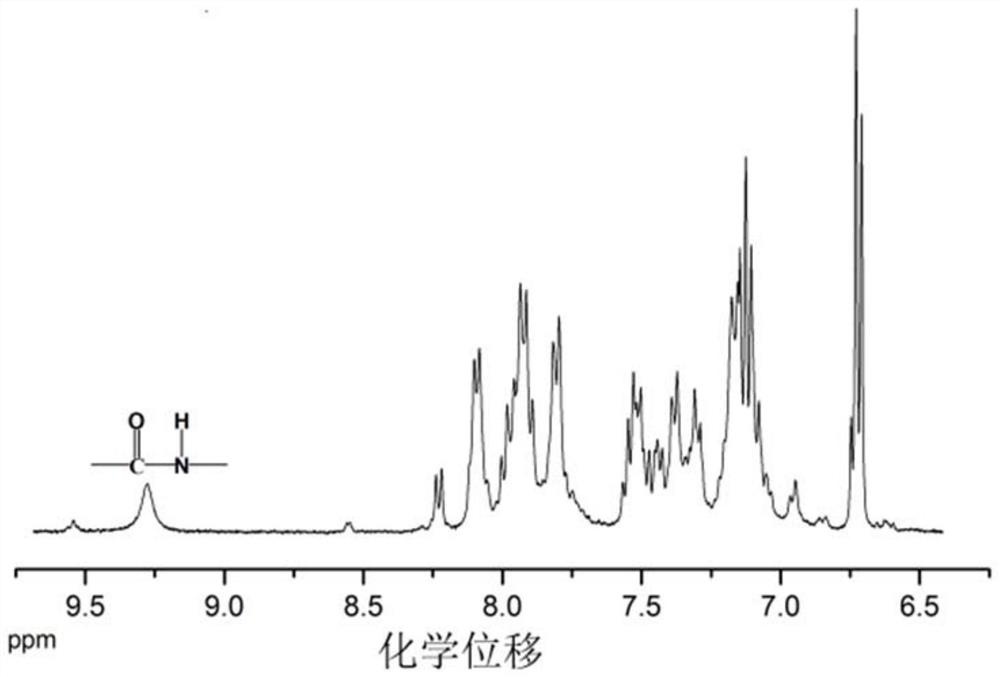
[0048] Specific Embodiments 1. In this embodiment, a multifunctional triarylamine-based polyamide containing a fused-ring naphthyl side group structure is a multifunctional triarylamine-based polyamide P1 containing a fused-ring naphthyl side group structure. Triarylamine-based polyamide P2 with naphthyl side group structure or multifunctional triarylamine-based polyamide P3 with fused ring naphthyl side group structure;
[0049] The structural formula of the multifunctional triarylamino polyamide P1 containing fused ring naphthyl side group structure is:
[0050] In the formula, n is an integer of 7 to 20;
[0051] The structural formula of the multifunctional triarylamine-based polyamide P2 containing a fused-ring naphthyl side group structure is:
[0052] In the formula, n is an integer of 7 to 23;
[0053] The structural formula of the multifunctional triarylamino polyamide P3 containing fused ring naphthyl side group structure is:
[0054] In the formula, n is an...
specific Embodiment approach 2
[0055] Specific embodiment two, the preparation method of a kind of multifunctional triarylamino polyamide containing fused ring naphthyl side group structure of this embodiment is carried out according to the following steps:
[0056] (1) Under a nitrogen atmosphere, add solvent DMSO to p-bromoaniline and cesium fluoride, under stirring and constant pressure conditions, add p-fluoronitrobenzene at a rate of 1-2 drops per second, and then heat up Perform constant temperature reaction at 110°C, cool to room temperature after the reaction is complete, place the reaction product in distilled water at 24-25°C, stir until the crude product precipitates, then filter the crude product, and wash the crude product with water at 99-100°C 2 ~3 times, then placed in a vacuum drying oven for drying, then recrystallized with acetic acid, then filtered out the crystalline product, and vacuum-dried the crystalline product to obtain a yellow powder, which was 4-bromo-N, N-bis( 4-nitrophenyl) a...
specific Embodiment approach 3
[0067] Specific embodiment three: the difference between this embodiment and specific embodiment two is: if the diacid is 2,2-bis(4-carboxyphenyl)hexafluoropropane, then the multifunctional triaryl with fused ring naphthyl side group structure The amino polyamide is a multifunctional triaryl amino polyamide P1 containing a fused ring naphthyl side group structure. Others are the same as in the second embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Coloring time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com