Method for preparing hexamethylenediamine from cyclohexene
A technology of cyclohexene and hexamethylene diamine, which is applied in chemical instruments and methods, preparation of amino-substituted functional groups, preparation of carbonyl compounds by oxidation, etc., can solve the problems of high toxicity of adiponitrile, dependence on imports, high price, etc., to improve the choice performance, catalyst stability, route cleaning effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
[0111]The preparation of embodiment 1-10 supported type W, Mo catalyst
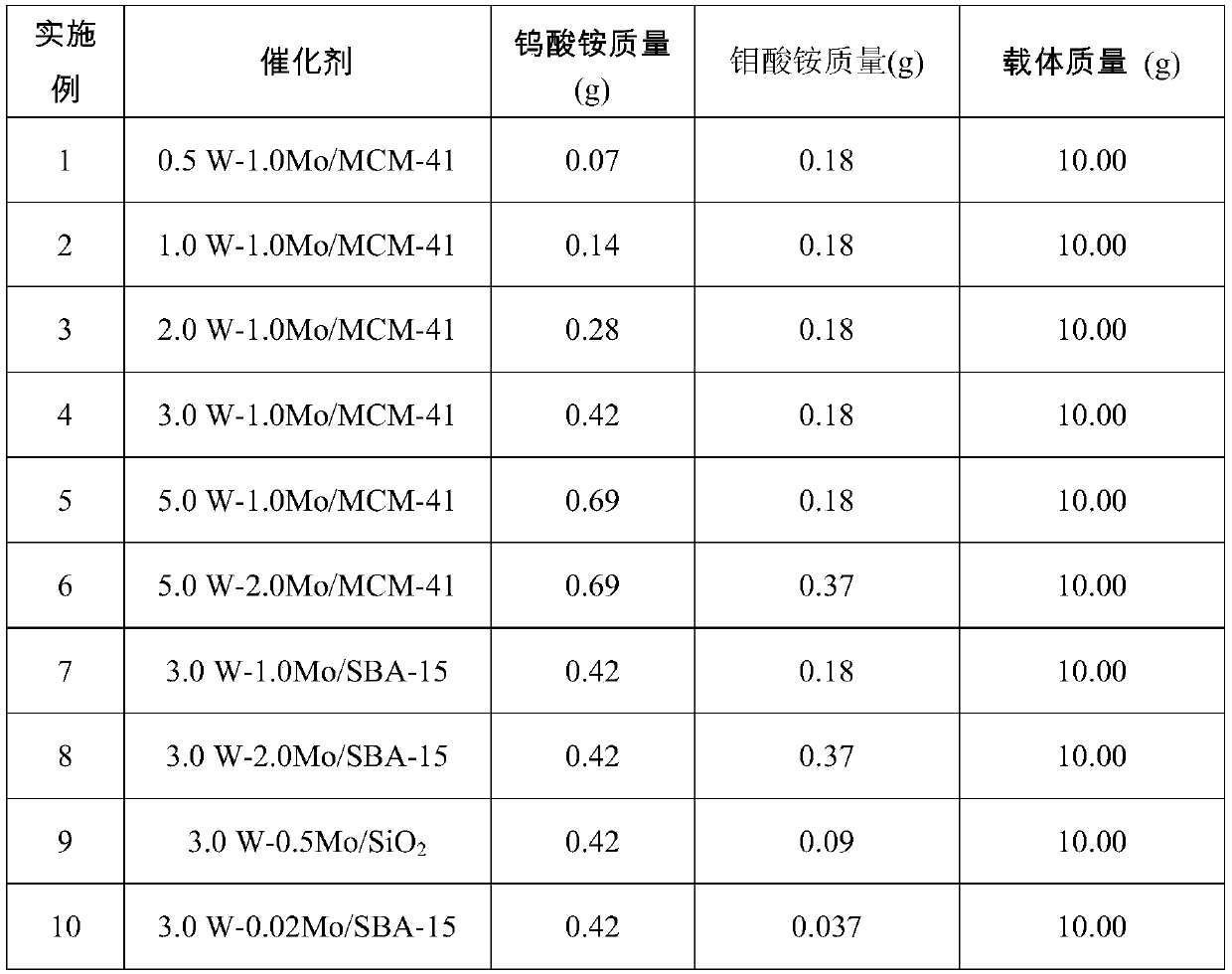
[0112] Firstly, the carrier was calcined at 550 °C for 4 h in a nitrogen atmosphere to remove species adsorbed in the pores. Dissolve a certain amount of ammonium tungstate and ammonium molybdate in water, and set the volume according to the saturated water absorption of the carrier (when using MCM-41, set the volume to 50 ml, when using SBA-15, set the volume to 30 ml, use medium When using porous silica, set the volume to 20 ml), take 10 g of the carrier and use the equal volume impregnation method to load the metal salt solution on the carrier, then place it in an oven at 100 ° C for 12 h, and then bake it in a muffle furnace at 500 ° C for 4 h Prepare the catalyst. The types and qualities of carriers and metal salts are shown in Table 1.
[0113] The preparation parameter of table 1 catalyst
[0114]
[0115] Wherein, in the name of the catalyst aW-bMo / X, a represents the mass loading of the met...
Embodiment 11
[0116] Example 11 Cyclohexene oxidation to adipaldehyde
[0117] In a 250mL round-bottomed flask, add 1.0g of cyclohexene, 0.10g of methyl pyruvate, 25mL of acetonitrile, 0.5W-1.0Mo / MCM-41 catalyst (0.10g), rise to 20°C, and feed ozone with a concentration of The mixed gas of 100mg / L, described mixed gas is made up of ozone and oxygen, and described mixed gas flow rate is 80mL / min, is down to room temperature rapidly after reacting 1h, analyzes product composition with gas chromatograph.
Embodiment 12-20
[0118] Example 12-20 Cyclohexene oxidation to adipaldehyde
[0119] The cyclohexene oxidation reaction was carried out according to the same steps as in Example 11, and the specific differences and results of the reaction conditions are shown in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com