Preparation method and use method of magnetic porous biochar for removing chromium in water
A biochar and water removal technology, applied in chemical instruments and methods, water pollutants, biofuels, etc., can solve the problems of easy agglomeration, difficult recovery, inactivation, etc., and achieve easy separation, multiple effective sites, and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] A preparation method of magnetic porous biochar that removes chromium in water, using Fe(III) as a magnetic precursor, slow pyrolysis and in-situ carbon reduction after impregnation and loading, to prepare Artemia egg shells into magnetic porous biochar carbon.
[0050] The biomass raw material of the biochar is Artemia eggshell, which belongs to the waste of the aquatic industry, has low cost, and can realize waste utilization at the same time; the magnetism is derived from nano-Fe 3 o 4 Particles, Nano Fe 3 o 4 The average particle size is about 70nm, and its precursor is Fe(III). During the slow pyrolysis process of egg shells, part of Fe(III) is reduced to Fe(II) in situ by the generated carbon without additional Adding other reducing agents, the preparation method is simple;
[0051] The porous structure is a microscopic multi-level channel structure with a pore size of 200nm-2μm, nano-Fe 3 o 4 The particles are evenly distributed in the multi-level channels....
Embodiment 1
[0066] Weigh 5g of Artemia egg shells washed with ethanol solution and soak in 200mL of 1mol / L FeCl 3 The solution was stirred at 25°C for 24h, then the solid was filtered out and dried at 60°C.
[0067] The above-mentioned dried solids were placed in a tube furnace, and after nitrogen protection, the temperature was raised to 450°C at a heating rate of 12.5°C / min, and the heating was stopped after keeping for 5 hours to complete slow pyrolysis and in-situ carbon reduction. process.
[0068] After the material was cooled to room temperature, it was ground, rinsed with deionized water, and dried at 60°C to obtain magnetic porous biochar.
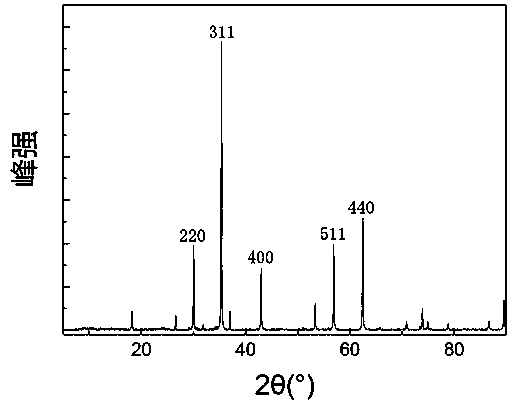
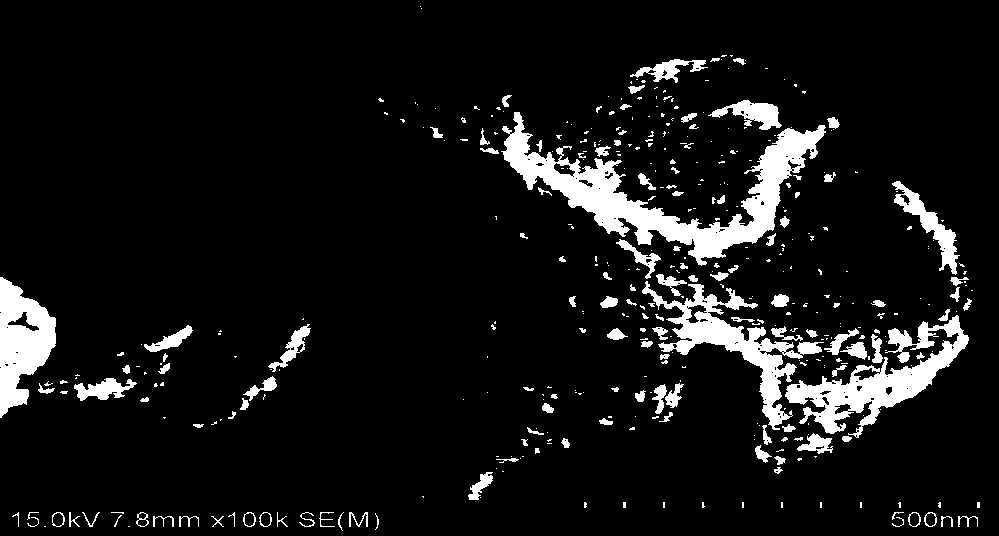
[0069] Such as figure 1 As shown, the magnetic porous biochar retains a complete multi-level pore structure, and the nanoparticles are evenly distributed in the pores. 3 o 4 form exists, figure 2 Its XRD characteristic diffraction peak.
Embodiment 2
[0071] Take 0.025 g of the magnetic porous biochar prepared in Example 1, and add it to K with an initial Cr(VI) concentration of 50 mg / L. 2 Cr 2 o 7 solution, the volume of the solution was 50 mL, and the pH was adjusted to 2.0 with NaOH and HCl. The above system was shaken at 160 rpm for 24 hours at 25°C. After the reaction, the biochar was magnetically separated, and the remaining chromium ion concentration in the liquid phase was measured by diphenylcarbazide spectrophotometry. The calculated Cr(VI) adsorption amount was 143.89 mg / g, and the removal rate was 94.26%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com