Biotin and cell-penetrating peptide co-mediated breast cancer targeted intelligent liposome material
A technology targeting liposomes and breast cancer, applied in the field of medicine, can solve problems such as high cell and tissue toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
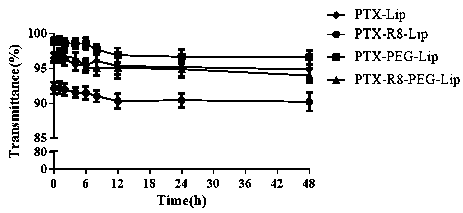
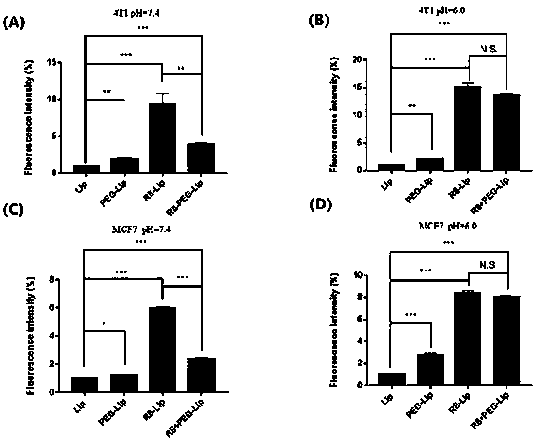
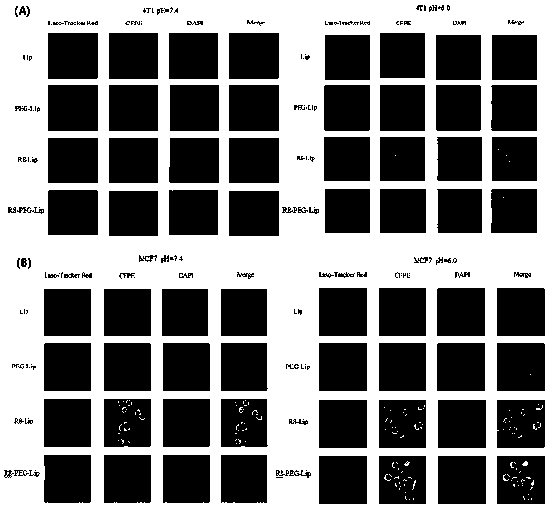
Image
Examples
Embodiment 1
[0033] Example 1 Preparation of Compound 2
[0034]
[0035] Weigh monobenzyl succinate (2.84 g, 13.62 mmol) into a double-necked flask, replace argon three times, dissolve with 55 mL of anhydrous dichloromethane, stir at -5°C, and add chloroformic acid under argon protection After addition of isobutyl ester (IBCF, 1.86 g, 13.62 mmol) and N-methylmorpholine (NMM, 1.38 g, 13.62 mmol), the activation was continued for 30 min. Compound 1 (1.50 g, 11.35 mmol) was dissolved in 2.5 mL of anhydrous dichloromethane and added dropwise to the above reaction solution. After the dropwise addition was completed, the reaction was carried out at room temperature for 3 h, and the completion of the reaction was monitored by TLC. The solvent in the reaction system was removed by concentration under reduced pressure, and the residue was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain 2.77 g of a colorless transparent oil, with a yield of 75.70%. ...
Embodiment 2
[0036] Example 2 Preparation of Compound 3
[0037]
[0038] Compound 2 (2.25 g, 6.98 mmol) was dissolved in 16 mL of degassed methanol, 330 mg of palladium on carbon was added, hydrogen was replaced 5 times, and stirred at room temperature. The completion of the reaction was monitored by TLC. The reaction liquid was filtered through diatomaceous earth, and the diatomaceous earth was washed 2-3 times with methanol. The filtrate was collected and concentrated under reduced pressure to remove the solvent to obtain 1.60 g of a colorless oil with a yield of 98.70%. The product was directly carried on to the next step without purification. 1 H-NMR (400 MHz, DMSO- d 6 , ppm) δ : 1.38 (s, 9H), 2.29 (t, J = 6.0 Hz, 2H), 2.41 (t, J = 6.0 Hz, 2H), 8.68 (s, 1H), 9.52 (s, 1H), 11.0 (brs, 1H).
Embodiment 3
[0039] Example 3 Preparation of Compound 5
[0040]
[0041] Cholesterol 4 (30.00 g, 77.59 mmol) was dissolved in 100 mL of anhydrous pyridine, and a pyridine solution (50 mL) of p-toluenesulfonyl chloride (TsCl, 23.67 g, 124.14 mmol) was slowly added dropwise at 0 °C. After the dropwise addition was completed, the reaction solution was moved to 55° C. to react overnight. TCL monitored the completion of the raw material reaction, and distilled off pyridine under reduced pressure, dissolved the residue with ethyl acetate (300 mL), and washed it with dilute hydrochloric acid (1 N, 100 mL × 2), saturated aqueous sodium chloride solution (100 mL × 2 ), dried over anhydrous sodium sulfate, filtered, and the solvent was removed from the filtrate under reduced pressure to obtain 37.79 g of a white solid, with a yield of 90.06%. The product was directly subjected to the next reaction without purification. Mp: 129-132°C (Literature Mp: 130-132°C).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com