Bacillus coagulans strain and application thereof
A technology of bacillus coagulans and strains, applied in the field of microbiology, can solve the problems of unreachable effects and discounted probiotic effects, and achieve the effects of favorable spore formation, high spore formation rate, and good heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
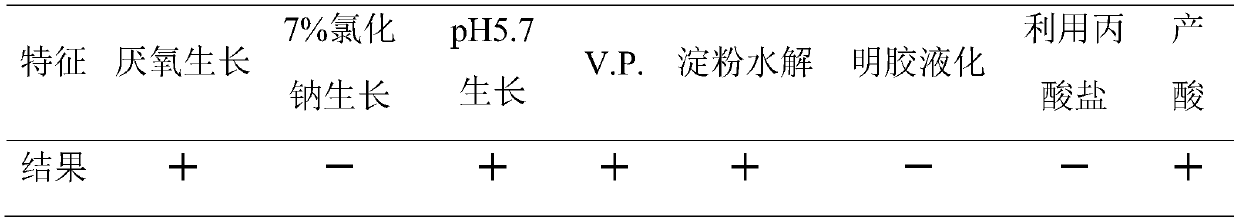
[0059] Hangzhou Baoankang Biotechnology Co., Ltd. screened out a strain of Bacillus coagulans (Bacillus coagulans), whose preservation number is CGMCC No. 17374, which is a new strain.
[0060] Its fermentation process is as follows:
[0061] (1) Primary slant culture of Bacillus coagulans:
[0062] The original bacteria were inoculated on the primary slant medium, and cultured at 30°C-45°C for 20-48h. The primary slant medium is a mixed solution (g / L) prepared according to the following ratio: yeast powder 6.0-15.0 g / L peptone 6.0-15.0 g / L glucose 2.0-10.0 g / L sodium chloride 5.0-10.0 g / L dipotassium hydrogen phosphate 1.0~5.0g / L manganese sulfate 0.1~3.0g / L pH 6.6~7.2
[0063] (2) Secondary culture of Bacillus coagulans:
[0064] Inoculate the single colony of the primary Bacillus coagulans in the secondary culture medium in the step (1), the liquid volume is 50-100mL / 200mL / 300mL / 500mL, and cultivate under the conditions of 30°C-45°C and 150-300rpm / min After 20-48 hours,...
Embodiment 2
[0074] Bacillus coagulans strain (Bacillus coagulans), its preservation number is CGMCC No.17374, the application of this strain in the direction of microbial inoculation agent.
[0075] details as follows:
[0076] (1) Collect the solid fermentation product of Bacillus coagulans in Example 1 and dry it at a temperature of 40-60°C for 18-36 hours until the water content reaches 5-10%.
[0077] (2) The dried product in step (1) is pulverized, and the particles are passed through a 60-mesh sieve, collected and bagged, sealed and stored in a cool and dry place.
[0078] (3) Determining the amount of viable Bacillus coagulans and the number of spores of the microbial agent in step (2).
[0079] (4) Based on the measurement results in step (3), the powder in step (2) is quantitatively packed into the capsule shell, and the quantification of live bacteria is 1.5×10 8 ~3.0×10 8 CFU / capsule.
[0080] The above-mentioned capsules are commercially available medicinal capsule shells....
Embodiment 3
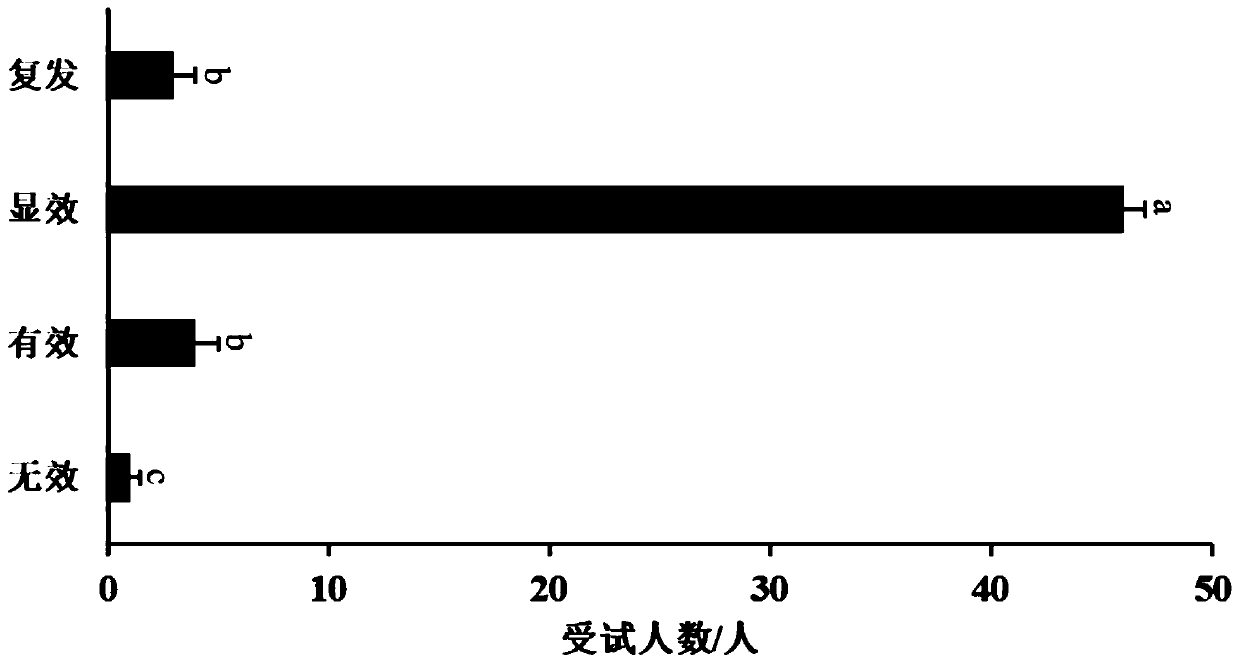
[0083] The microbial preparation capsule described in Example 2 was tested for biological efficacy.
[0084] 1. Subject volunteers 50 people voluntarily participated in this research and signed the informed consent.
[0085] Inclusion criteria:
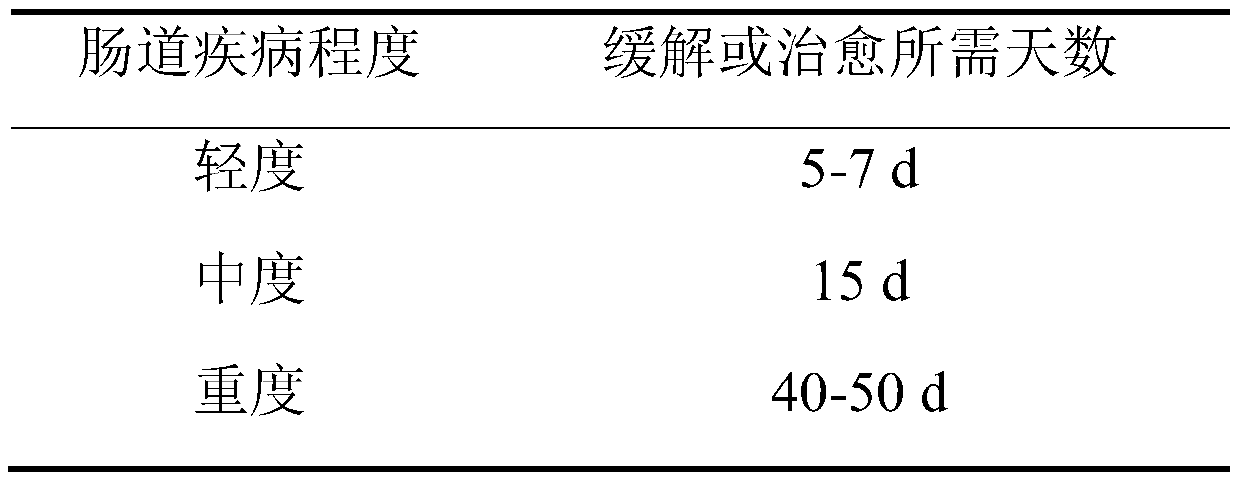
[0086] Inclusion criteria: (1) have long-term or short-term constipation, laxative-dependent constipation, seasonal or common diarrhea, alcoholic colitis, abdominal distension and other symptoms of intestinal diseases; (2) aged 8 to 60 years old; (3) voluntarily participate in this Study, signed informed consent.
[0087] 2. Method
[0088] 1. Research method: The volunteers were tested for symptoms of intestinal diseases, and 50 people who met the inclusion criteria were selected, and they were given the Bacillus coagulans microbial preparation capsules described in Application Example 1, and a comparative test was carried out for 60 days. And continue to pay attention to the effect after stopping taking it.
[0089] 2. Evaluatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com