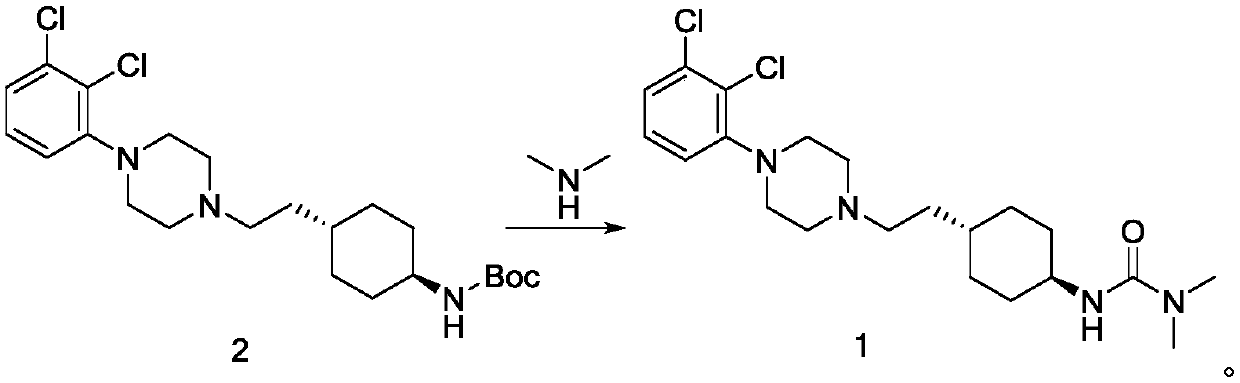
Preparation method of cariprazine
A technology of cariprazine and piperazine, which is applied in the field of preparation of cariprazine, can solve the problems of complex post-processing, low yield, and many by-products, and achieve simple post-processing, high yield, and low reaction by-products little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The preparation method of trans-1-(BOC-amino)-4-(2-hydroxyethyl)cyclohexane preparation formula compound 3 is: add trans-1-(BOC-amino)-4 to a 500ml single-necked bottle -(2-Hydroxyethyl)cyclohexane 20g (0.082mol), add 200ml dichloromethane to dissolve, add triethylamine 12.5g (0.123mol), add p-toluenesulfonyl chloride 18.76g (0.0984mol) under stirring, room temperature Stir the reaction for 20 hours, TLC (ratio of developing agent: dichloromethane: methanol = 10:1) to confirm the completion of the reaction, add 200ml of water to the reaction system, stir to dissolve, separate the layers, separate the water phase, and concentrate the organic phase Until there is almost no flow, add 200ml of n-hexane to the concentrate, stir for 2 hours, filter, rinse the filter cake with n-hexane, bake under reduced pressure at 50-60°C for 8-10 hours, and obtain 26.5g of white solid, with a yield of 81.3%;
[0062] Wherein the formula compound 3 1 H NMR data are:
[0063] δ7.76-7.78(d,...
Embodiment 2
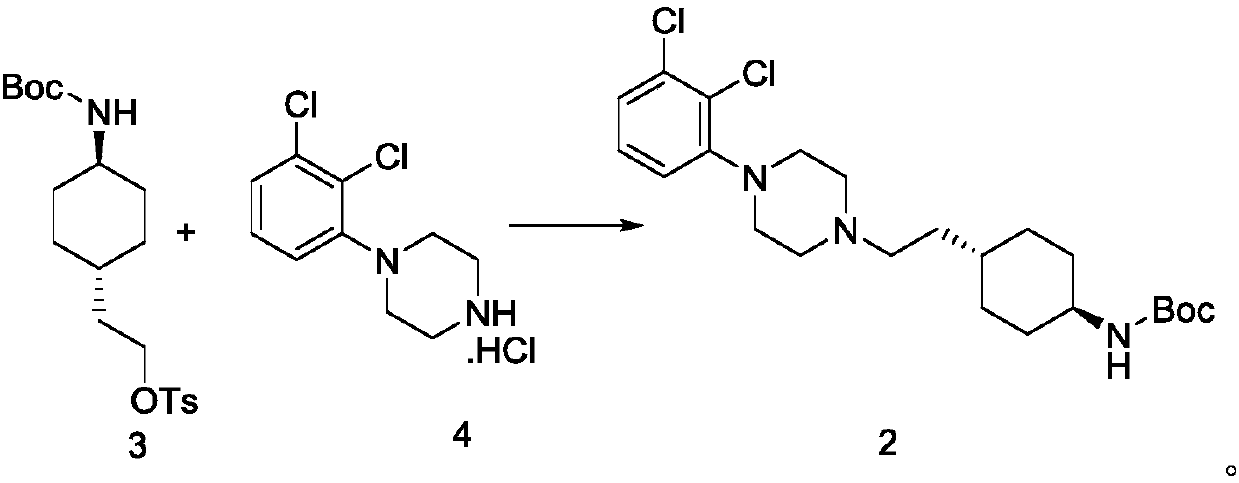
[0065] Preparation of formula compound 2 trans N-tert-butoxycarbonyl-4-(2-(4-(2,3-dichlorophenyl)-piperazin-1-yl)-ethyl)-cyclohexylamine
[0066]
[0067] Add 33.7g 1-(2,3-dichlorophenyl)piperazine hydrochloride (formula compound 4), 400g ethanol, 27.2g potassium carbonate, 50g trans 2-(1-(4- (N-tert-butoxycarbonyl)-amino)-cyclohexyl)-ethyl-4-methylbenzenesulfonate (formula compound 3), reacted at 75°C for 12-18h, cooled to room temperature after the reaction, and added to the reaction Add 400g of water to the system and stir for 2h, filter, rinse the filter cake with ethanol, and dry at 50°C to constant weight to obtain 63g of trans-N-tert-butoxycarbonyl-4-(2-(4-(2,3-dichloro Phenyl)-piperazin-1-yl)-ethyl)-cyclohexylamine;
[0068] Wherein the 1H NMR data of formula compound 2 is:
[0069] δ10.7(s, 1 H),7.33-7.38(m,2H),7.18-7.21(m,1H),6.67-6.69(d,1H),3.40-3.57(m,2H),3.15-3.33(m9H),1.70-1.77( t,4H), 1.61-1.62(t,2H), 1.37(s,9H), 1.21-1.23(d,4H), 1.14-1.18(d,2H).
Embodiment 3
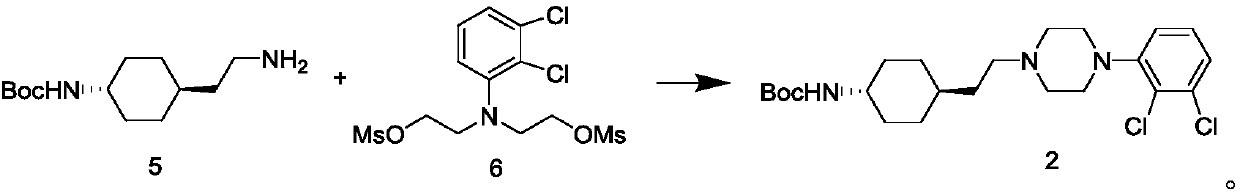
[0071] The preparation of formula compound 2
[0072]
[0073] Add 21g (0.052mol) of compound 6 crude product to the reaction flask, add 12.6g (0.052mol) of compound 5, potassium carbonate 14.4g (0.104mol), acetonitrile 200ml, reflux and stir to react for 12h, the central control reaction is completed, and add to the reaction system Add 200ml of water, stir for 1 hour, filter, rinse the filter cake with water and acetonitrile respectively, bake the solid at 50-60°C for 6 hours, and obtain 18.8g of compound 2 as a white solid, with a yield of 79.3%;
[0074] Wherein the 1H NMR data of formula compound 2 is:
[0075] δ10.7(s, 1 H),7.33-7.38(m,2H),7.18-7.21(m,1H),6.67-6.69(d,1H),3.40-3.57(m,2H),3.15-3.33(m9H),1.70-1.77( t,4H), 1.61-1.62(t,2H), 1.37(s,9H), 1.21-1.23(d,4H), 1.14-1.18(d,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com