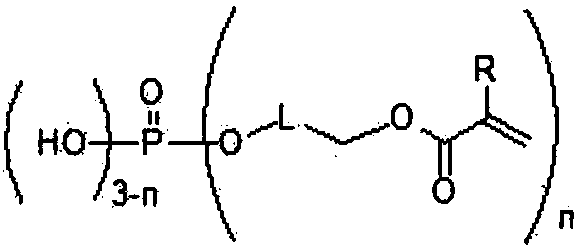
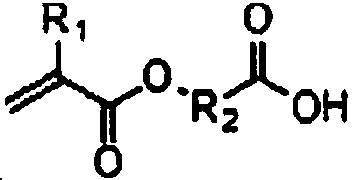
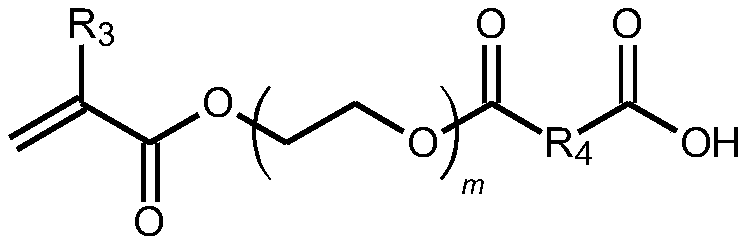
Quantum dots and application thereof
A quantum dot and carbon number technology, applied in the field of image display devices, can solve the problems of decreased stability and reliability, inability to solve problems such as light resistance, decreased dispersibility, etc., to achieve improved brightness and reliability, excellent oxidation stability, The effect of less peeling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0485] Synthesis Example 1: Synthesis of InP / ZnS Core-Shell Quantum Dots
[0486] 0.05839 g of indium acetate, 0.12019 g of oleic acid, and 10 mL of 1-octadecene (ODE) were added to a three-necked flask (3-neck flask). While stirring the above-mentioned flask, after degassing at 110° C. and 100 mTorr for 30 minutes, it was heated to a temperature of 270° C. under an inert gas until the solution became transparent.
[0487] As a phosphorus (P) precursor, prepare 0.025054 g of tris(trimethylsilyl)phosphine, add 0.5 mL of 1-octadecene and 0.5 mL of tri-n-octylphosphine, and stir it under an inert gas Quickly inject into the above-mentioned flask which has been heated to 270°C. After reacting for 1 hour, the reaction was rapidly cooled to complete the reaction. Then, when the temperature of the flask reached 100° C., 10 mL of toluene was injected, and then transferred to a 50 mL centrifuge tube. After adding 10 mL of ethanol, purification was performed twice by precipitation an...
Synthetic example 2
[0491] Synthesis Example 2: Synthesis of InP / ZnSe / ZnS Core-Shell Quantum Dots
[0492] Add 0.4 mmol (0.058 g) of indium acetate (0.058 g), 0.6 mmol (0.15 g) of palmitic acid (0.15 g) and 20 mL of 1-octadecene into the reactor, and heat to 120° C. under vacuum. After 1 hour, the atmosphere in the reactor was switched to nitrogen. After heating to 280° C., a mixed solution of 0.2 mmol (58 μl) of tris(trimethylsilyl)phosphine (TMS3P) and 1.0 mL of trioctylphosphine was quickly injected and reacted for 0.5 minutes.
[0493]Next, 2.4 mmol (0.448 g) of zinc acetate, 4.8 mmol of oleic acid, and 20 mL of trioctylamine were added to the reactor, and heated to 120° C. under vacuum. After 1 hour, the atmosphere in the reactor was switched to nitrogen, and the temperature of the reactor was raised to 280°C. 2 mL of the previously synthesized InP core solution was added, followed by the addition of 4.8 mmol of selenium (Se / TOP) in trioctylphosphine, and then the final mixture was allowed...
Synthetic example 3
[0495] Synthesis example 3: Cardo series alkali-soluble resin (BP-1)
[0496] (1) Add 9,9'-bis(4-glycyloxyphenyl)fluorene (Hearchem Company) 138g, 2-carboxyethyl acrylate (2-Carboxyethyl acrylate) as bisphenol epoxy compound in the reactor ) 54g, benzyltriethylammonium chloride (Oi Kakin Co.) 1.4g, triphenylphosphine (Aldrich (Aldrich) company) 1g, propylene glycol methyl ethyl acetate (Daicel Chemical (Daicel Chemical) ) company) 128g and hydroquinone 0.5g, the temperature was raised to 120°C and maintained for 12 hours, thereby synthesizing the compound represented by the following chemical formula 25.
[0497] (2) Add 60 g of compounds represented by the following chemical formula 25, 11 g of biphenyltetracarboxylic dianhydride (Mitsubishi Gas (Mitsubishi Gas) company), 3 g of tetrahydrophthalic anhydride (Aldrich Company), propylene glycol in the reactor 20 g of methyl ethyl acetate (Daicel Chemical Co., Ltd.) and 0.1 g of N,N'-tetramethylammonium chloride were heated to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com