An acid-responsive cross-linked polymer prodrug and its preparation method and application
A cross-linked polymer technology, which is applied in pharmaceutical formulation, drug combination, drug delivery, etc., can solve the problems of low delivery efficiency and drug leakage, achieve good biocompatibility, small side effects, and overcome the problems of easy leakage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1P
[0055] The synthesis of embodiment 1PEG-2VEA-HCPT
[0056] (1) Synthesis of small molecule vinyl ether acrylate (VEA)
[0057] The synthetic route of small molecule VEA is as follows:
[0058]
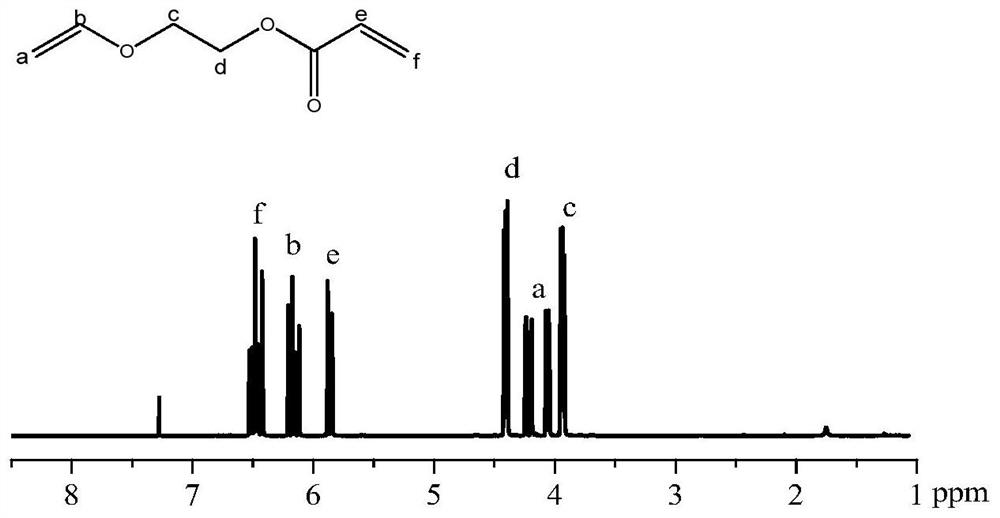
[0059] Ethylene glycol monovinyl ether (150ml, 1.73mol) was dissolved in 1.2L of dichloromethane, and 310mL of triethylamine (TEA) was added, and acryloyl chloride (168mL, 1.67mol) was added dropwise in an ice bath. After the dropwise addition, react at room temperature for 8h. After the reaction, the reaction liquid was extracted three times with aqueous sodium carbonate solution, and the organic phase was dried over anhydrous magnesium sulfate, filtered, concentrated under reduced pressure, and dried in vacuo for 6 h. The concentrated product was distilled under reduced pressure, and the fraction with a steam temperature of 56°C was collected to obtain a colorless liquid with a pungent odor, vinyl ether acrylate, with a yield of 63.2%. Proton NMR spectrum such as figure 1 show...
Embodiment 2P
[0068] The synthesis of embodiment 2PEG-2VEMA-HCPT
[0069] (1) Synthesis of small molecule vinyl ether methacrylate (VEMA)
[0070] The synthetic route of the small molecule compound VEMA is as follows:
[0071]
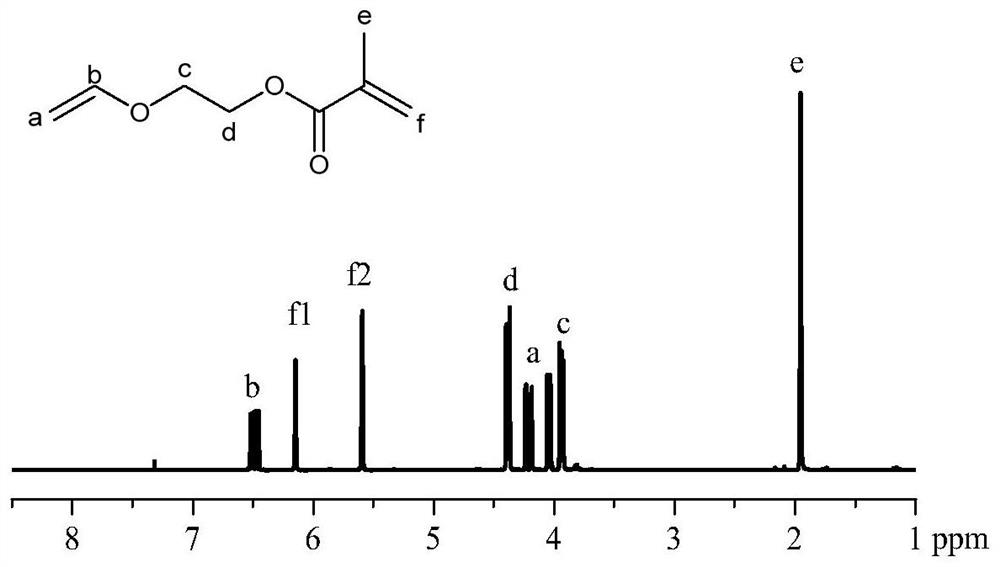
[0072] Ethylene glycol monovinyl ether (150ml, 1.73mol) was dissolved in 1.2L of dichloromethane, and 310mL of triethylamine (TEA) was added, and methacryloyl chloride (168mL, 1.49mol) was added dropwise in an ice bath . After the dropwise addition, react at room temperature for 8h. After the reaction, the reaction liquid was extracted three times with aqueous sodium carbonate solution, and the organic phase was dried over anhydrous magnesium sulfate, filtered, concentrated under reduced pressure, and dried in vacuo for 6 h. The concentrated product was distilled under reduced pressure, and the fraction with a steam temperature of 58°C was collected to obtain a colorless liquid with a pungent odor, vinyl ether methacrylate, with a yield of 51.6%. Proton NMR s...
Embodiment 3
[0081] The preparation of embodiment 3 polymer prodrug micelles (PEG-2VEA-HCPT)
[0082] Polymer prodrug micelles (PEG-2VEA-HCPT) were prepared by solvent exchange method. Under ultrasonic conditions, 0.1 mL of ethanol solution (20 mg / mL) of polymer prodrug PEG-2VEA-HCPT was slowly added to 2 mL of high-purity water, and the resulting mixed solution was continued to be ultrasonicated for half an hour. The assembled micelles were mixed with photoinitiator I2959 (mass fraction of polymer prodrug 5%), and dialyzed in high-purity water for 2 hours after ultraviolet irradiation for 15 minutes. Figure 5 is the particle size characterization diagram of polymer prodrug micelles (PEG-2VEA-HCPT) before and after crosslinking. The results showed that the average particle size of prodrug micelles before cross-linking was 180nm, and the particle size distribution was 0.22; after cross-linking, the average particle size of the micelles was 170nm, and the particle size distribution was 0.1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com