Glucoamylase MhglaA from myceliophthora heterothallica, gene for coding glucoamylase MhglaA, and application of glucoamylase MhglaA to glucose production
A technology of glucoamylase and myceliophthora, applied in the field of genetic engineering and biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
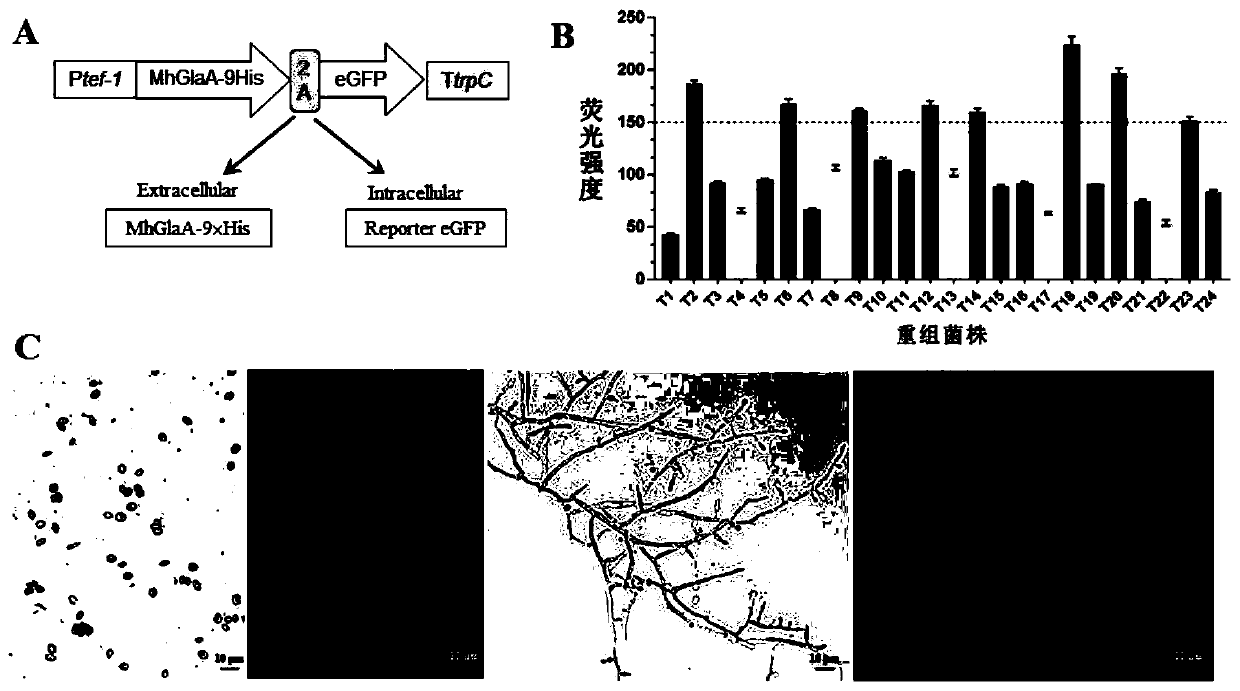
[0034] Example 1. Construction of FMDV 2A peptide-mediated system for recombinant expression of glucoamylase MhGlaA from Myceliophthora thermophila
[0035] 1. Construction of glycoamylase MhGlaA recombinant expression vector
[0036]The plasmid pAN52-bar (Gu SY, Li JG, Chen BC, Sun T, Liu Q, Xiao DG, TianCG. Metabolic engineering of the thermophilic filamentous fungus Myceliophthora thermophila to produce fumaric acid. Biotechnology for Biofuels. 2018, 11: 323.) was used as Backbone constructs expression vectors. Using Myceliophthora thermophila glucoamylase (Mycth_72393; XuGB, Li JG, Liu Q, Sun WL, Jiang M, Tian CG. Transcriptional analysis of Myceliophthora thermophila on soluble starch and role of regulator AmyR on polysaccharide degradation. Bioresource technology.2018, 265: 558-562.) sequence as a reference, the analysis and comparison of biological information in the protein sequence encoded by Myceliophthora heterothaliana revealed the glucoamylase MhGlaA (Myche_75600...
Embodiment 2
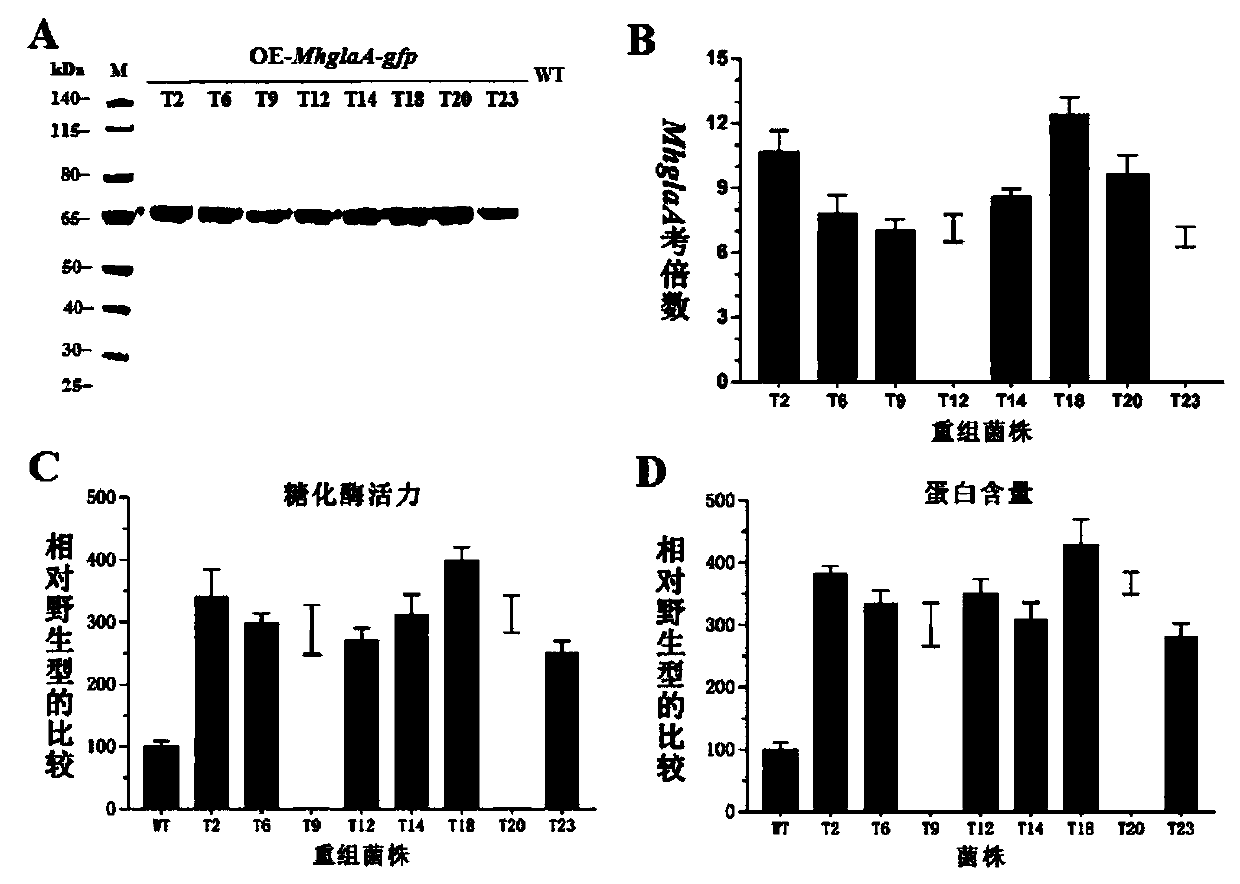
[0073] Example 2, Phenotype Analysis of Glucoamylase Production by Myceliophthora thermophila Recombinant Strain
[0074] 1. Western blot detection of MhGlaA secretion in the recombinant strain of Myceliophthora thermophila
[0075] The 8 recombinant strains OE-MhglaA-gfp (T2, T6, T9, T12, T14, T18, T20, T23) with strong eGFP fluorescence expression and the wild-type strain WT were induced to produce glucoamylase, and the induction culture conditions were : Cultivate in 2% (2g / 100mL) water-soluble starch medium (recipe: 50×Vogel's salt 2mL, water-soluble starch 2g, peptone extract 0.5g, constant volume to 100mL, autoclaved) at 45°C and 150rpm for 3d , the sample was centrifuged to take the supernatant, and the recombinant protein MhGlaA-9×His was detected by Western blot. The primary antibody used was His-Tag rabbit monoclonal antibody, and the secondary antibody was rabbit anti-IgG HRP antibody. The result is as figure 2 As shown in A, the recombinant protein MhGlaA-9×His ...
Embodiment 3
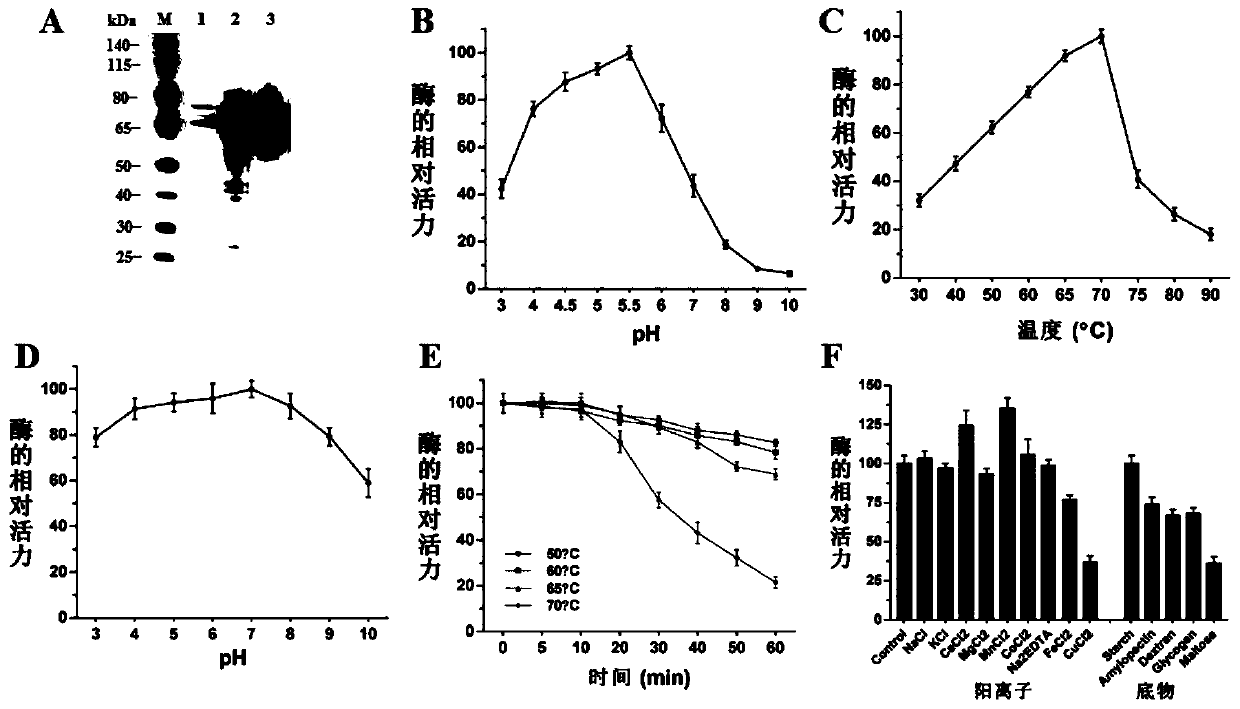
[0087] Example 3. Preparation of recombinant glucoamylase rMhGlaA and research on its enzymatic properties
[0088] 1. Preparation of glucoamylase rMhGlaA by fermentation
[0089] Place the recombinant strain T18 producing the highest level of glucoamylase on 2% (2g / 100mL) water-soluble starch medium (recipe: 50×Vogel's salt 2mL, water-soluble starch 2g, constant volume to 100mL, autoclaved) at 45°C For induction culture, the fermented liquid cultured for 5 days was taken at 4°C and centrifuged at 12,000×g for 30 min, the supernatant was collected and concentrated by 50kD ultrafiltration, and the protein concentrate was purified according to Qiagen’s Ni-NTA Matric operation manual.
[0090] Use buffer A (20mmol / L NaH 2 PO 4 , 500mmol / L NaCl and 20mmol / L imidazole, pH7.4) to equilibrate the column, the sample flows through the Ni-NTA purification column at a flow rate of 1mL / min, and then buffer B (20mmol / L sodiumphosphate, 500mmol / L NaCl and 500mmol / L Li imidazole (pH 7.4) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com