D-A type aromatic ring conjugated dendritic ring metal iridium complex and application thereof
An iridium complex, D-A technology, applied in indium organic compounds, platinum group organic compounds, compounds containing elements of group 8/9/10/18 of the periodic table, etc., can solve the problem of less types of near-infrared electroluminescent materials, etc. It can reduce the dipole-induced intermolecular interaction, increase the distance, and improve the hole transport performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] D-A Cyclometallic Iridium Complex with N,N-Diphenylamine as Dendritic Unit ((DPA) 2 -TPA-BTz-Iq) 2The preparation of Irpic, the synthetic route is as follows (the raw material 4′,4′-bis(N,N-diphenylamino)-4-bromotriphenylamine CAS: 105389-36-4, purchased from Zhengzhou Alpha Chemical Co., Ltd.) :
[0035]
[0036] Preparation of Compound 1
[0037] In a 100mL single-necked flask, add 272mg (0.41mmol) 4′,4′-bis(N,N-diphenylamino)-4-bromotriphenylamine, 208mg (0.82mmol) pinacol diborate, 161mg (1.64mmol) potassium acetate (KOAc), 7mg (0.01mmol) [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride, 50mL 1,4-dioxane, extracted Vacuum N 2 Under protection, the temperature was controlled at 80°C for 24 hours. Stop the reaction, after cooling to room temperature, pour the reaction solution into 100mL distilled water, extract with DCM (3×30mL), and wash the organic layer with anhydrous MgSO 4 Dry for 6h. After filtration, the solvent was distilled off under redu...
Embodiment 2
[0049] The present invention also provides the characterization and photophysical and electrochemical performance tests of the involved D-A aromatic ring conjugated dendritic ring metal iridium complex:
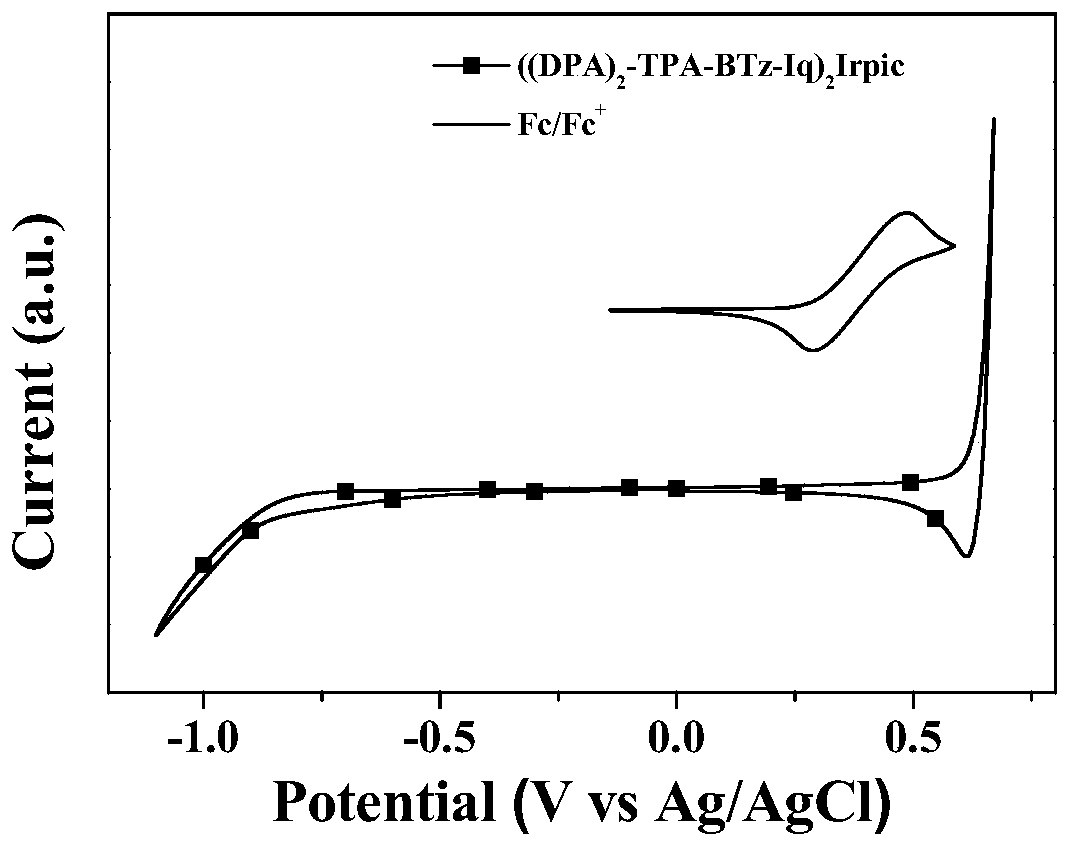
[0050] Nuclear magnetic resonance (NMR) spectra were recorded at 298K on a Bruker DRX 500 spectrometer in deuterated chloroform or deuterated acetone solutions with tetramethylsilane as an internal standard. Time-of-flight mass spectrometry measurements were performed on a Bruker Bifiex III MALDI-TOF. UV-Vis absorption and photoluminescence spectra were measured using a Varian Cray 50 absorption spectrometer and a Perkin-Elmer LS50B luminescence spectrometer. Cyclic voltammetry (CV) was performed on a CHI 600E electrochemical workstation at room temperature and under argon at 100mV·S -1 The scan rate is carried out, the electrolyte solution is n-Bu 4 NPF 6 (0.1M) acetonitrile solution; Pt sheet, Pt wire and Ag / AgCl electrode were used as working electrodes respectively; fo...
Embodiment 3
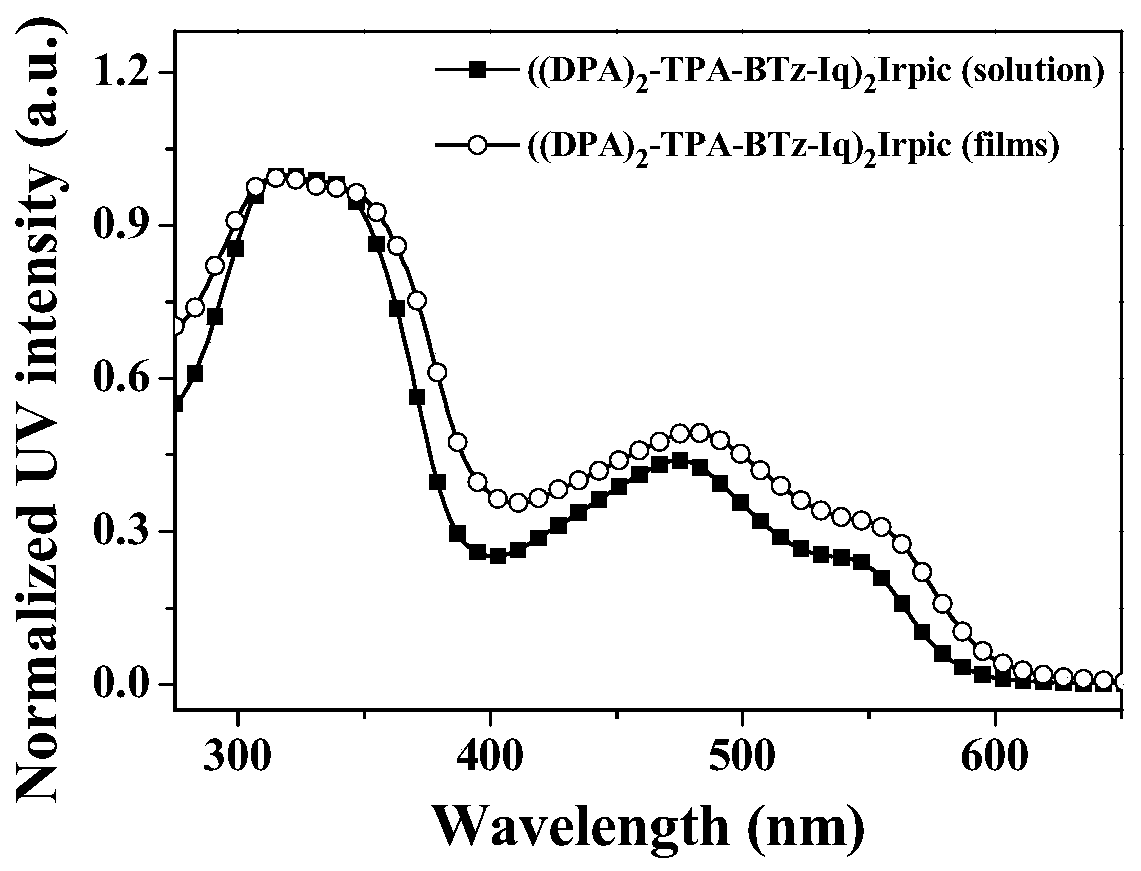
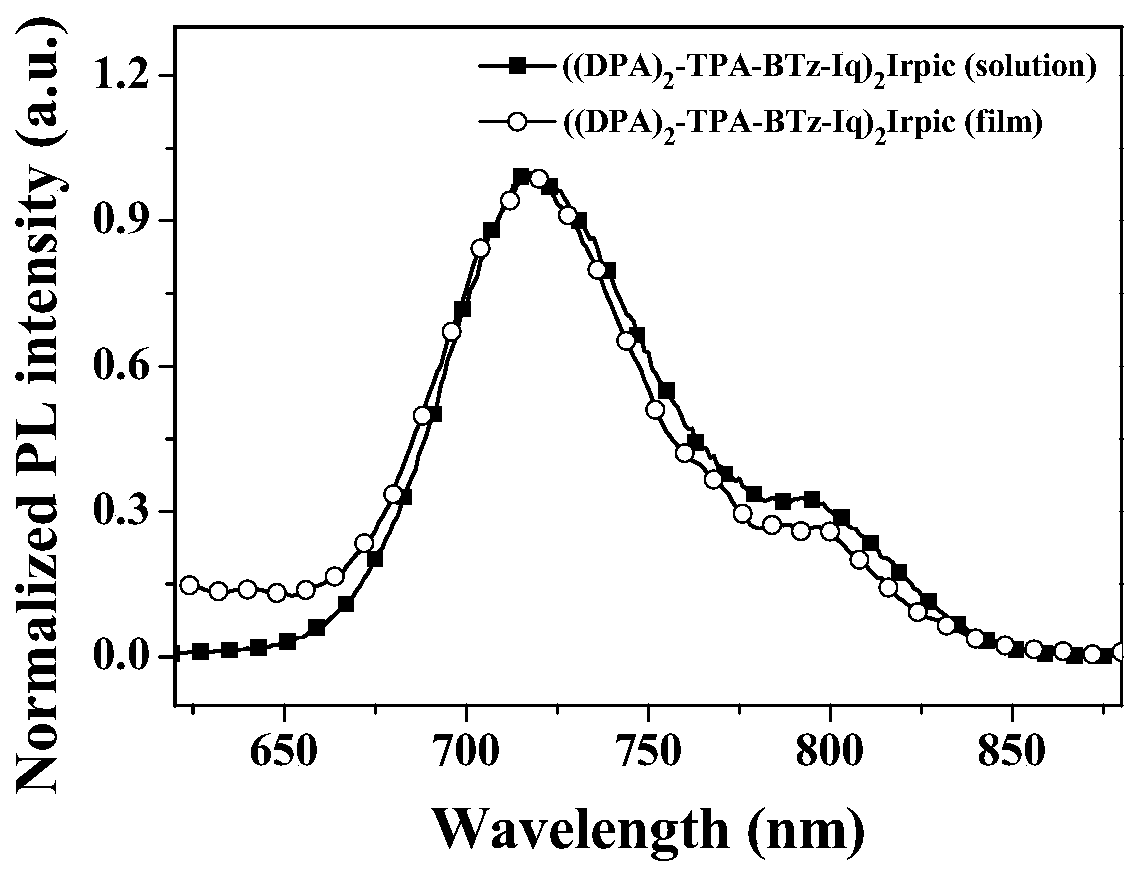
[0052] Complex ((DPA) 2 -TPA-BTz-Iq) 2 UV-Vis Absorption Spectrum of Irpic
[0053] The complex ((DPA) 2 -TPA-BTz-Iq) 2 Irpic was dissolved in dichloromethane to make 10 -5 M solution or prepared as a solid film, test its UV-Vis absorption spectrum. figure 1 For the complex ((DPA) 2 -TPA-BTz-Iq) 2 UV-Vis absorption spectra of Irpic in dichloromethane (DCM) solution and solid film. Depend on figure 1 It can be seen that the complex ((DPA) 2 -TPA-BTz-Iq) 2Irpic shows similar and strong UV-visible absorption peaks in dichloromethane solution and solid film. The absorption peaks are located at 318nm, 473nm, and 543nm in dichloromethane solution, and the absorption peaks are located at 318nm, 481nm, and 553nm in solid film. The absorption peak of the iridium complex at 290-350nm is assigned to π-π * Electronic transition absorption; the absorption peak at 450-550nm is attributed to metal-ligand charge transfer transition (MLCT) absorption; in addition, the absorption pea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com