Thermally activated delayed fluorescence material and organic light-emitting device
A technology of organic light-emitting devices and thermal activation delay, which is applied in the fields of light-emitting materials, organic chemistry, and electric solid-state devices. It can solve the problems of short life and scarcity of materials, and achieve high glass transition temperature, low driving voltage, and high thermal stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
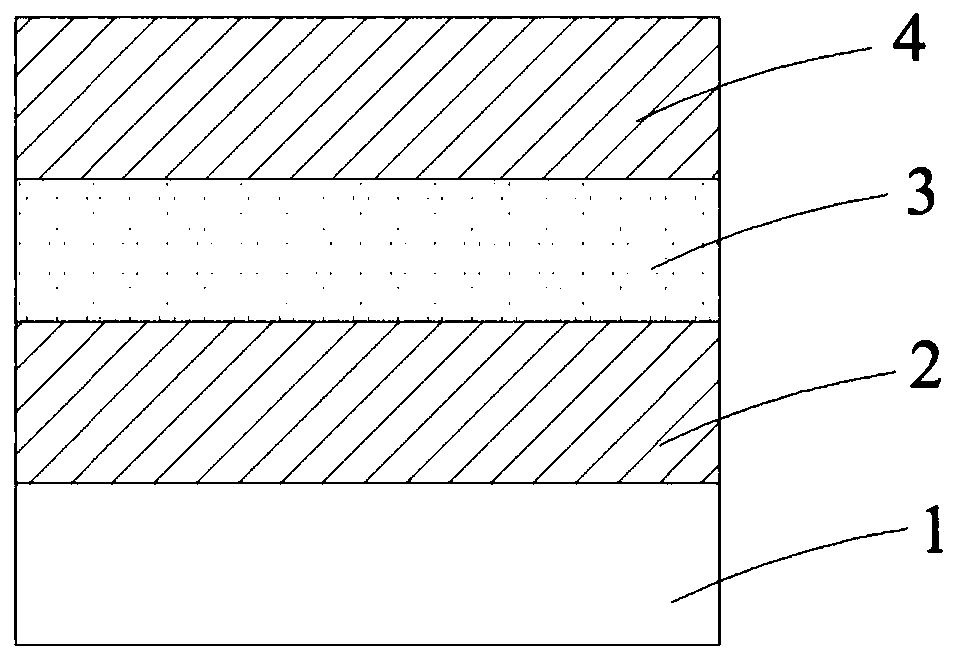
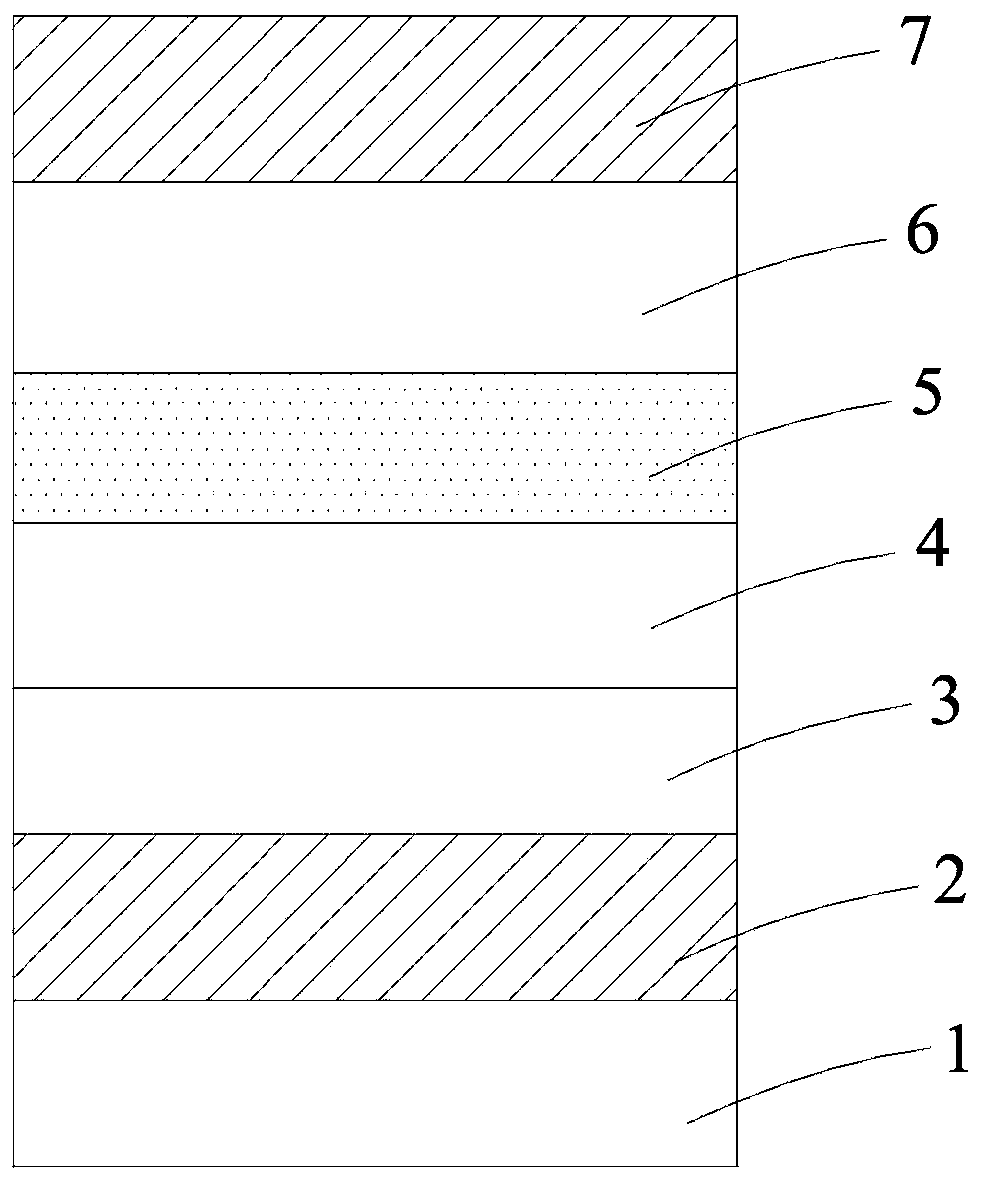
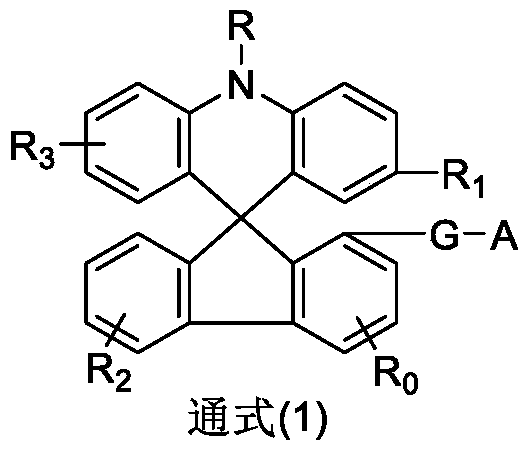
Image
Examples
preparation example 1
[0237] Synthesis of the following compound 1
[0238]
[0239] Under nitrogen protection, 9-fluorenone-1-boronic acid (5.0g, 22.32mmol), 2-chloro-4,6-diphenyl-1,3,5-triazine (5.98g, 22.32mmol), Tetrakis(triphenylphosphine)palladium (773.39mg, 0.67mmol) and anhydrous potassium carbonate (6.16g, 44.64mmol) were placed in a 250ml round bottom flask, and 90ml of tetrahydrofuran and 22ml of distilled water were added. The above mixture was heated to reflux for 24 hours. After the reaction was completed, the temperature was lowered to room temperature, filtered with suction, washed with a large amount of distilled water, and purified by recrystallization with dichloromethane / ethanol to obtain intermediate 1 (7.89g, 19.20mmol yield about 86%), which was dried for later use. Under the protection of nitrogen, put 2-bromo-N-methyl-N-phenylaniline (5.03g, 19.20mmol) in a 250ml two-necked bottle, add 77ml of anhydrous tetrahydrofuran to dissolve, and then place it at minus 78 degrees...
preparation example 2
[0241] Synthesis of the following compound 2
[0242]
[0243] Under the protection of nitrogen, put 2-bromotriphenylamine (6.22g, 19.20mmol) in a 250ml two-necked bottle, add 77ml of anhydrous tetrahydrofuran to dissolve, then place it at minus 78 degrees Celsius, and add 2.4M concentration of n- Butyllithium solution (8.80ml, 21.12mmol) was stirred at minus 78 degrees Celsius for 1 hour, then Intermediate 1 (7.89g, 19.20mmol) was added, stirred overnight, and then quenched by adding 20ml of distilled water. Remove tetrahydrofuran from the reaction solution under reduced pressure, add 40ml of dichloromethane to extract 3 times, remove the dichloromethane under reduced pressure, add ethanol for recrystallization, place the solid obtained after suction filtration and drying in a 250ml flask, add 100ml of acetic acid and stir for 10 Minutes later, 3ml of concentrated hydrochloric acid was added and heated to reflux at 110°C for 3 hours. After the reaction was completed, the...
preparation example 3
[0245] Synthesis of the following compound 3
[0246]
[0247] Under nitrogen protection, 9-fluorenone-1-boronic acid (5.0g, 22.32mmol), 2-(4-bromophenyl)-4,6-diphenyl-1,3,5-triazine (8.66 g, 22.32mmol), tetrakis(triphenylphosphine) palladium (773.39mg, 0.67mmol), anhydrous potassium carbonate (6.16g, 44.64mmol) were placed in a 250ml round bottom flask, and 90ml tetrahydrofuran and 22ml distilled water were added . The above mixture was heated to reflux for 24 hours. After the reaction was completed, the temperature was lowered to room temperature, filtered with suction, washed with a large amount of distilled water, and purified by recrystallization with dichloromethane / ethanol to obtain intermediate 2 (8.70 g, yield about 80%), which was dried for later use. Under the protection of nitrogen, put 2-bromo-triphenylamine (5.79g, 17.86mmol) in a 250ml two-necked bottle, add 77ml of anhydrous tetrahydrofuran to dissolve, then place it at minus 78 degrees Celsius, and add 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com