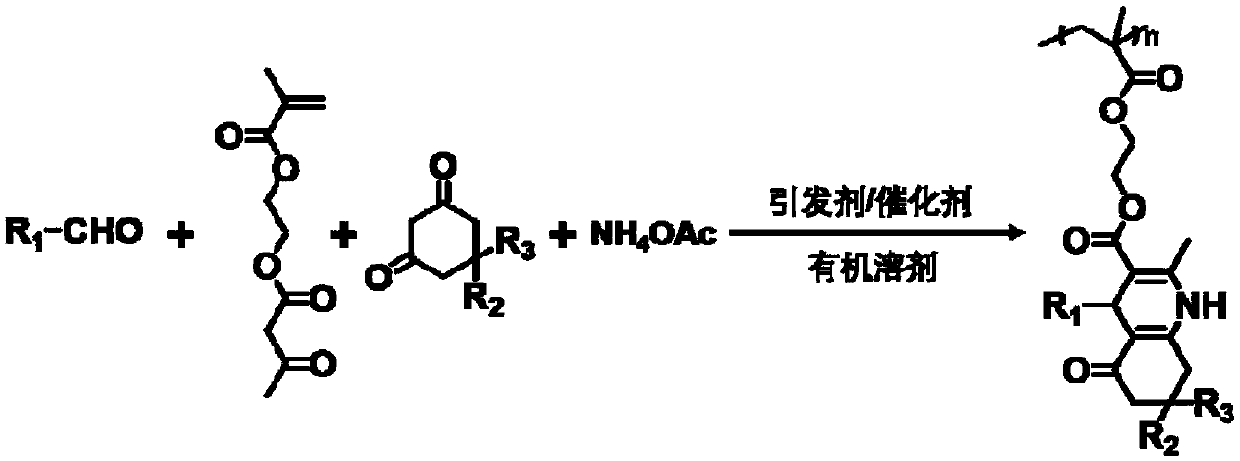
1, 4-dihydropyridine polymer as well as high-throughput preparation method and application thereof
A compound, C1-C3 technology, which is applied in the field of 1,4-dihydropyridine functional polymers and their high-throughput preparation, can solve the problems of many by-products, harsh reaction conditions, low yield and the like, and achieves simple operation. , Improve antibacterial ability, good universal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Prepare 1,4-dihydropyridine polymer from cinnamaldehyde, 5,5-dimethyl 1,3-cyclohexanedione, the steps are as follows:
[0057] 1) Add (397mg, 3mmol) cinnamaldehyde, (643mg, 3mmol) acetoacetate methacrylate (AEMA), (347mg, 4.5mmol) ammonium acetate and (421mg, 3mmol) to a 10mL polymerization tube, 5, 5-Dimethyl-1,3-cyclohexanedione, (of which cinnamaldehyde, ethylene glycol methacrylate acetoacetate, ammonium acetate, 5,5-dimethyl-1,3-cyclohexanedione Feeding molar ratio is 1:1:1.5:1) (15mg, 0.06mmol) azobisisoheptanonitrile (ABVN) and (30mg, 0.4mmol) glycine, 3mL of acetonitrile and 3mL of 1,4-dioxane ring as a solvent. After the solution was deoxygenated with nitrogen, it was placed in an oil bath at 75°C for reaction.
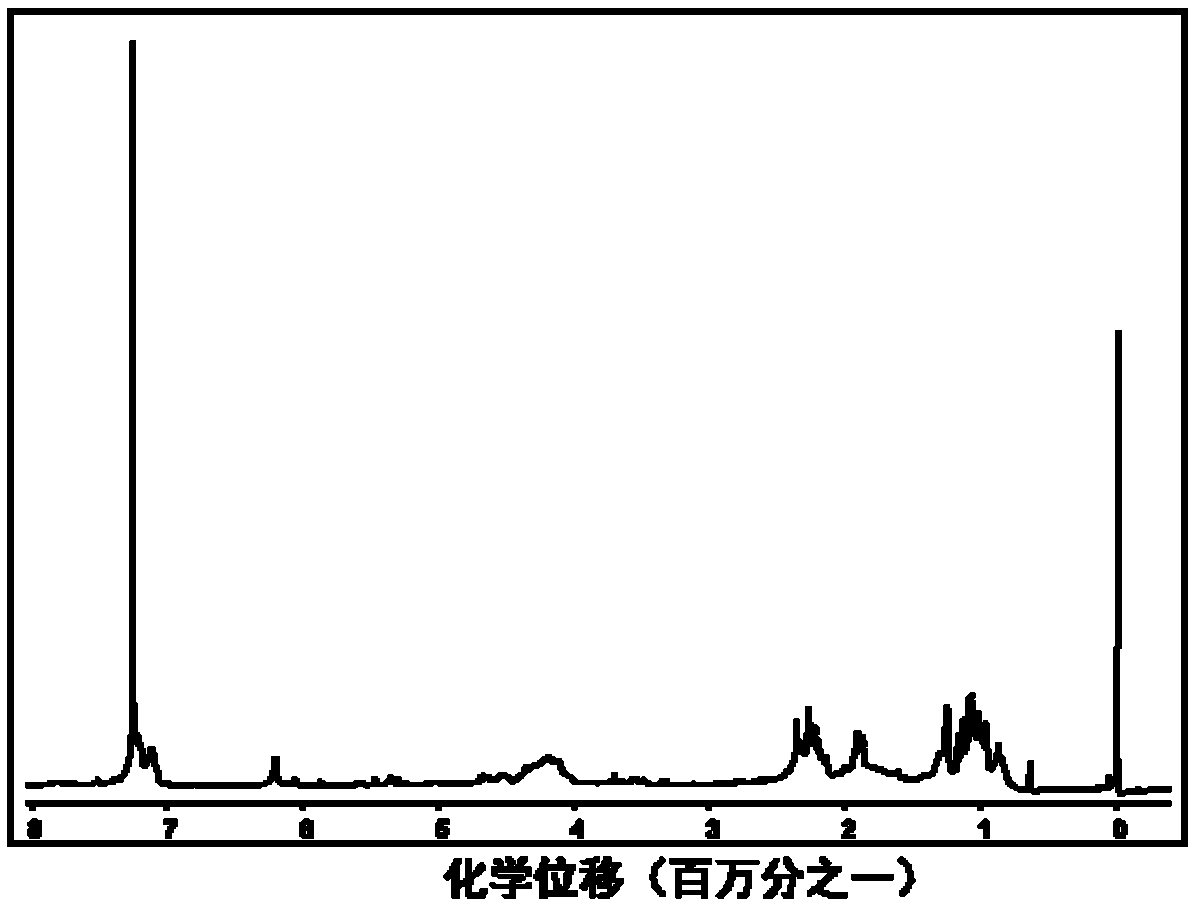
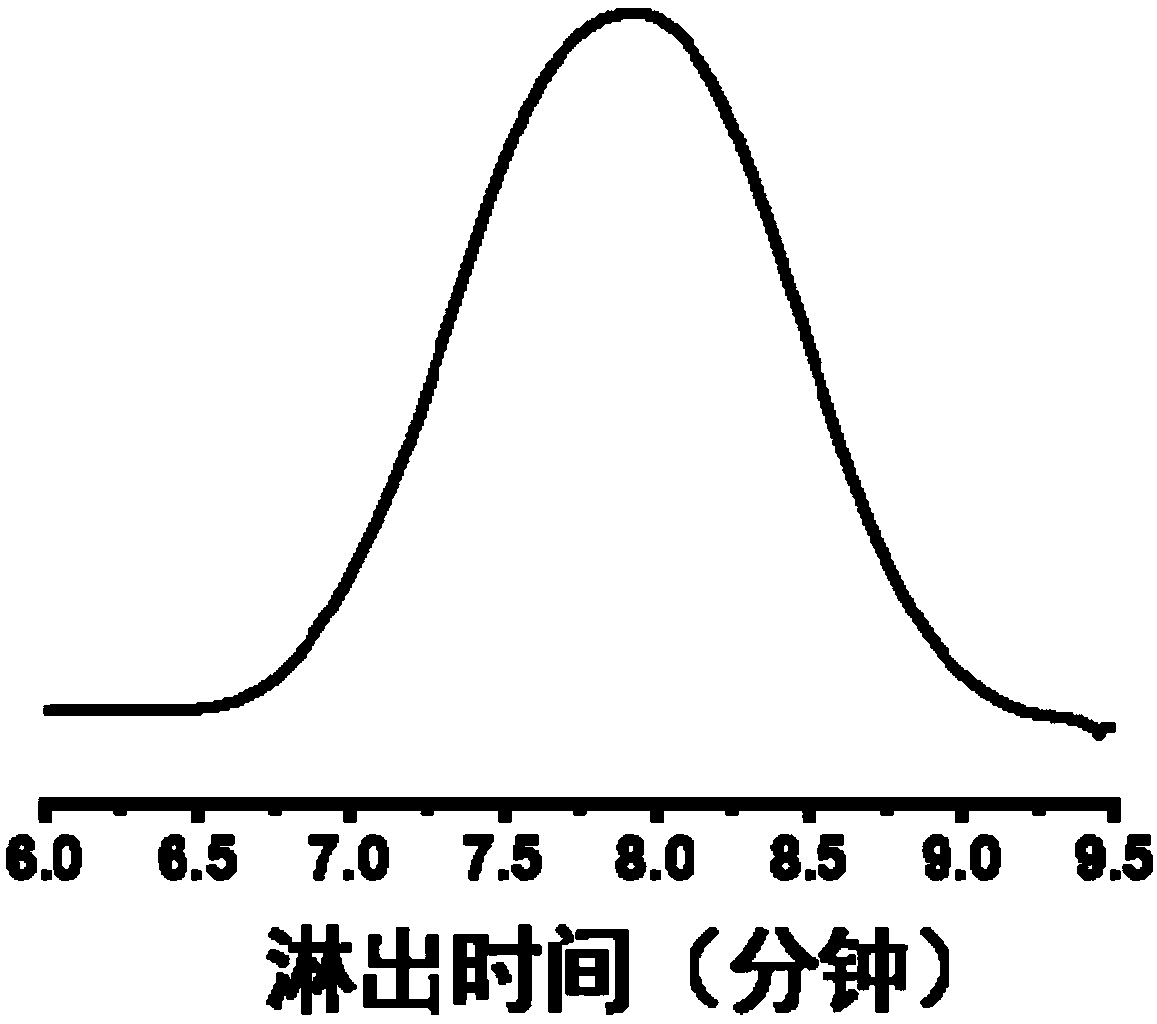
[0058] 2) Pour the mixture reacted in step 1) for 10 hours into deionized water to precipitate, centrifuge to collect the filter residue, dissolve the filter residue with 5mL THF, precipitate in ether, collect the yellow solid obtained by centrifugat...
Embodiment 2
[0062] Example 2. Preparation of 1,4-dihydropyridine polymers from citronellal and 1,3-cyclohexanedione, the steps are as follows:
[0063] 1) Add (463mg, 3mmol) citronellal, (643mg, 3mmol) acetoacetate methacrylate (AEMA), (347mg, 4.5mmol) ammonium acetate and (342mg, 3mmol) 1 ,3-cyclohexanedione, (wherein the molar ratio of cinnamaldehyde, acetoacetate methacrylate, ammonium acetate, 5,5-dimethyl-1,3-cyclohexanedione is 1:1 :1.5:1). (15 mg, 0.06 mmol) of azobisisoheptanonitrile (ABVN) and (30 mg, 0.4 mmol) of glycine, 3 mL of acetonitrile and 3 mL of 1,4-dioxane as solvents. After the solution was deoxygenated with nitrogen, it was placed in an oil bath at 75°C for reaction.
[0064] 2) Pour the mixed solution reacted for 10 hours in step 1) into deionized water to precipitate, collect the filter residue by centrifugation, dissolve the filter residue with 5mL tetrahydrofuran, and then precipitate in ether, collect the yellow solid obtained by centrifugation, and freeze-dry...
Embodiment 3、1
[0068] The antibacterial experiment of embodiment 3,1,4-dihydropyridine polymer antibacterial agent
[0069] 1. Blend 1.5 g of the 1,4-dihydropyridine polymer obtained in Example 1 with 3.0 g of polypropylene (PP) powder, and heat-press at 180° C. for 10 minutes to obtain a compressed tablet with a thickness of 0.45 mm.
[0070] 2. The above-mentioned compressed tablets were sterilized by irradiating with ultraviolet light for 30 minutes, and then put into a Erlenmeyer flask filled with 200 mL of sterile LB medium, and placed for use.
[0071] 3. Take 200 μL of seed solution (OD600=1.0) of Escherichia coli (E.coli) after RFP transfection, grow on a shaker (180 rpm, 37°C) for 72 hours, take it out and place it in paraformaldehyde (4%) for fixation, Confocal laser microscopy was used to observe the bacterial contamination, and the green light at 566 nanometers was used for excitation.
[0072] 4. According to the above steps, press 4.5 g of polypropylene into a tablet to obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com