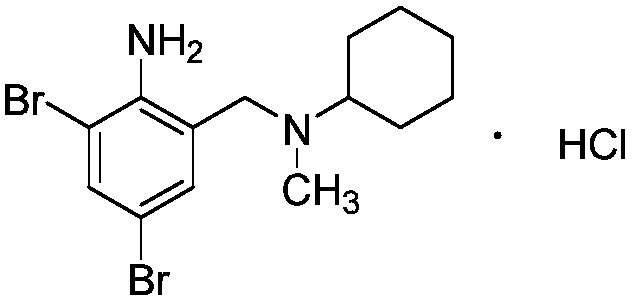
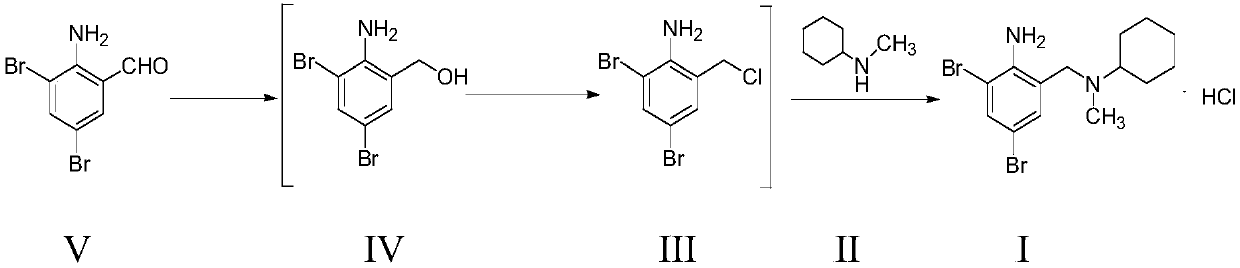
Preparation method of bromhexine hydrochloride
A technology of bromhexine hydrochloride and salt-forming reaction, which is applied in the preparation of organic compounds, preparation of amino compounds, chemical instruments and methods, etc., can solve the problems of large environmental pollution, easy decomposition, and demand, and achieve low environmental pollution and high product quality. The effect of high purity and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1 Bromhexine Hydrochloride Pilot Synthesis Experiment and Purification
[0067] Add 450g of 2-amino-3,5-dibromobenzaldehyde into 1.8kg of absolute ethanol and stir thoroughly. Control the temperature below 30°C and add 25g of sodium borohydride in batches, and stir at room temperature for 1 hour. Adjust the pH to 6-7 with hydrochloric acid, filter, add the filter cake to 1.8 kg of tetrahydrofuran to dissolve, dry to remove water, filter, and store the filtrate temporarily.
[0068] Add 356g of thionyl chloride and 2kg of tetrahydrofuran into another 5L reaction flask, and stir evenly. The temperature is controlled below 30°C, and the above filtrate is added dropwise to the system. After the dropwise addition was completed, the reaction was carried out at room temperature for 1 hour, and the system was concentrated under reduced pressure to remove tetrahydrofuran. After the concentration was complete, 2.7 kg of dichloromethane was added to the residue and stir...
Embodiment 2
[0073] Embodiment 2 bromhexine hydrochloride industrialized production and industrialized purification
[0074] Add 4.5kg of 2-amino-3,5-dibromobenzaldehyde into 18kg of absolute ethanol and stir thoroughly. Control the temperature below 30°C and add 0.25 kg of sodium borohydride in batches, and stir at room temperature for 1 hour. Adjust the pH to 6-7 with hydrochloric acid, filter, add the filter cake to 18 kg of tetrahydrofuran to dissolve, dry to remove water, filter, and store the filtrate temporarily.
[0075] Add 3.56kg of thionyl chloride and 20kg of tetrahydrofuran into another 100L reactor, and stir evenly. The temperature is controlled below 30°C, and the above filtrate is added dropwise to the system. After the dropwise addition was completed, the reaction was carried out at room temperature for 1 hour, and the system was concentrated under reduced pressure to remove tetrahydrofuran. After the concentration was complete, 27 kg of dichloromethane was added to the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com