Glucocorticoid-loaded nano-carrier as well as preparation and application thereof
A technology of glucocorticoids and nanocarriers, applied in the field of bioengineering, can solve problems such as poor targeting effect of lupus erythematosus, achieve good clinical application and transformation prospects, reduce expression, and simplify the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
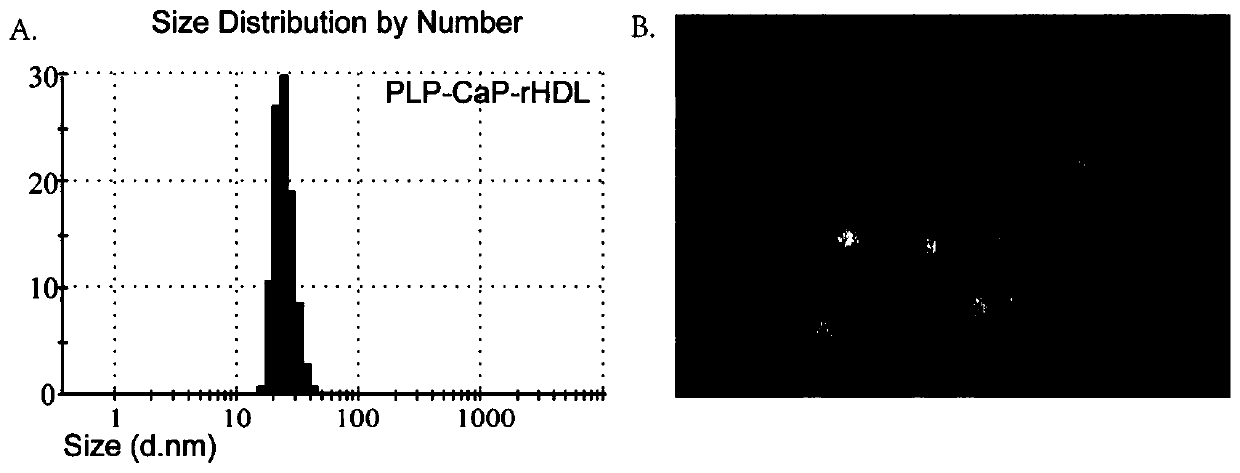
[0032] Example 1. Preparation and characterization of nanocarriers loaded with prednisolone sodium phosphate
[0033] (1) Preparation
[0034]Calcium phosphate core loaded with prednisolone disodium phosphate (PLP) was prepared by co-precipitation method:
[0035] The oil phase was formed from 14.5 ml cyclohexane and 6 ml Igepal CO-520. First, 600 μL of 2.5M CaCl 2 The solution was slowly added dropwise into 20mL oil phase to form a calcium phase. Then add 460μL 16.3mM Na 2 HPO 4 (a.q.) and 140μL of 60mM PLP (a.q.) mixture was slowly added dropwise to another 20mL oil phase to form a phosphorus phase, and 400μL of 20mM 1,2-oleoyl phosphatidic acid (1,2-dioleoyl phosphatidic acid, DOPA) solution was added . After stirring evenly, slowly add the calcium phase to the phosphorus phase, add 400 μL of 20mM DOPA, and mix and stir for 45 minutes. Subsequently, 40 mL of absolute ethanol was added to the above mixed microemulsion to break the emulsion. The mixture after demulsif...
Embodiment 2
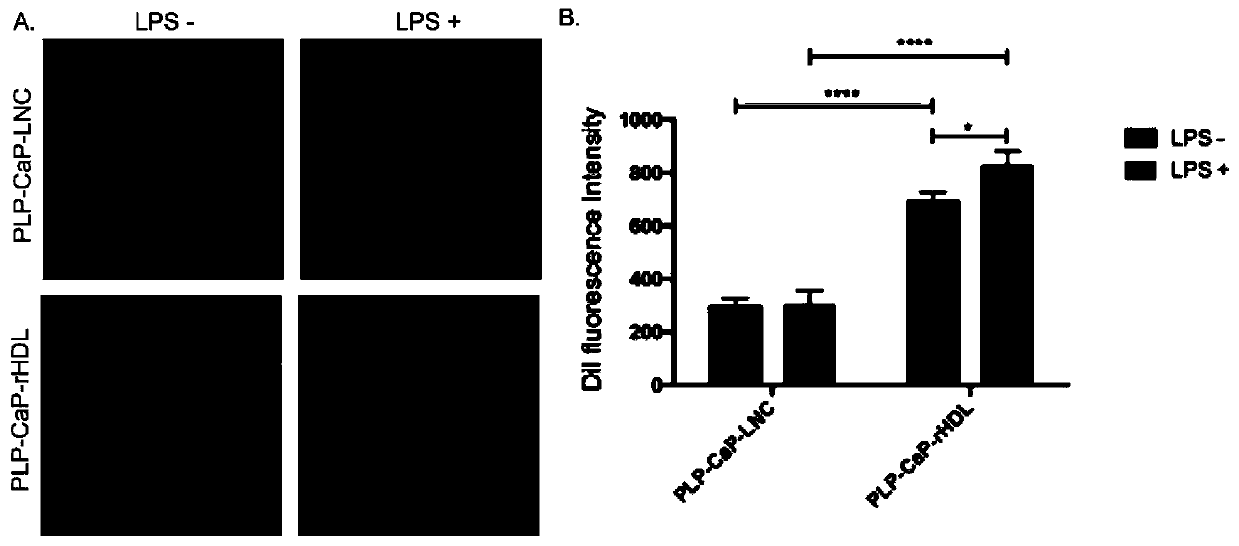
[0041] Example 2. Uptake of prednisolone sodium phosphate-loaded nanocarriers by LPS-stimulated mouse macrophage cell line RAW264.7
[0042] (1) Preparation
[0043] Adopt the film hydration method to prepare the common liposome of drug-loading with embodiment 1, add fluorescent dye DiI (20-100 μ g) simultaneously and put into 500ml round-bottomed flask, prepare the liposome of loading prednisolone sodium phosphate with embodiment 1 afterwards A recombinant high-density lipoprotein nano-drug delivery system is obtained to obtain a recombinant high-density lipoprotein nano-drug delivery system loaded with fluorescently labeled prednisolone sodium phosphate.
[0044] (2) LPS stimulates the mouse macrophage cell line RAW264.7 to establish an in vitro inflammation model
[0045] Cells were plated on 24-well culture plates in advance with a cell density of 5*10^4. After waiting for the cells to adhere to the wall (about 2 hours), 100ng / ml of LPS was added according to the experime...
Embodiment 3
[0049] Example 3. In vivo targeting effect of nanocarriers loaded with prednisolone sodium phosphate on systemic lupus erythematosus MRL / lpr mouse model
[0050] (1) Preparation
[0051] Similar to Example 2, a lipid nano drug delivery system modified with a functional penetrating peptide loaded with fluorescently labeled DiR was prepared.
[0052] (2) In vivo imaging experiment of small animals to observe the distribution in the body:
[0053] MRL / lpr mice and MRL / mpj mice were divided into PBS group, DiI-CaP-LNC group and DiI-CaP-rHDL group, respectively. The mice in each group were administered by tail vein injection, and 2 hours later, they were anesthetized by intraperitoneal injection of chloral hydrate, and the tissues (brain, heart, spleen, lung, double kidney, and double lower limbs) were taken and placed in an in vivo imager, using the Maestro in vivo imaging system Take tissue imaging pictures.
[0054] The mouse grouping and dosing regimen were consistent with t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com