Method for preparing enteric composite microcapsules by utilizing spray drying technology
A composite microcapsule, enteric-coated technology, applied in the directions of microcapsules, capsule delivery, medical preparations with inactive ingredients, etc., can solve the problems of difficult to achieve large-scale production, difficult process control, complicated operation, etc., to mask bad odors. , Effective release, high stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
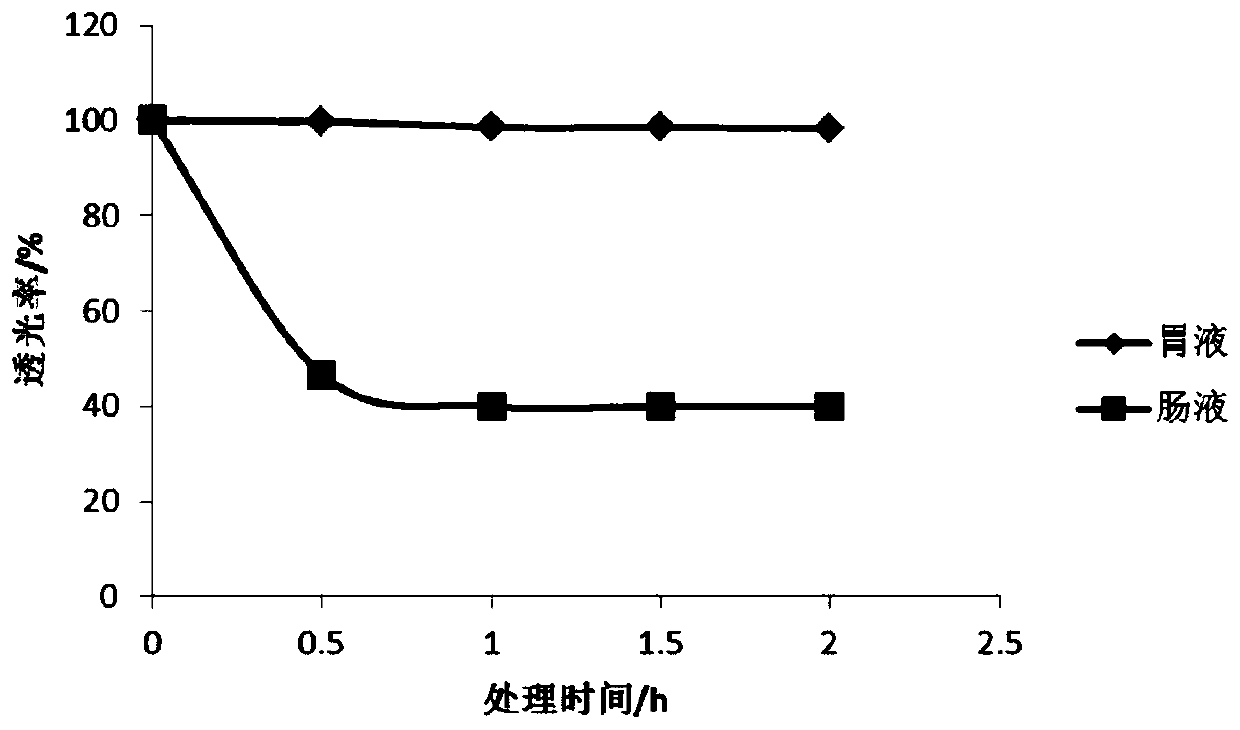
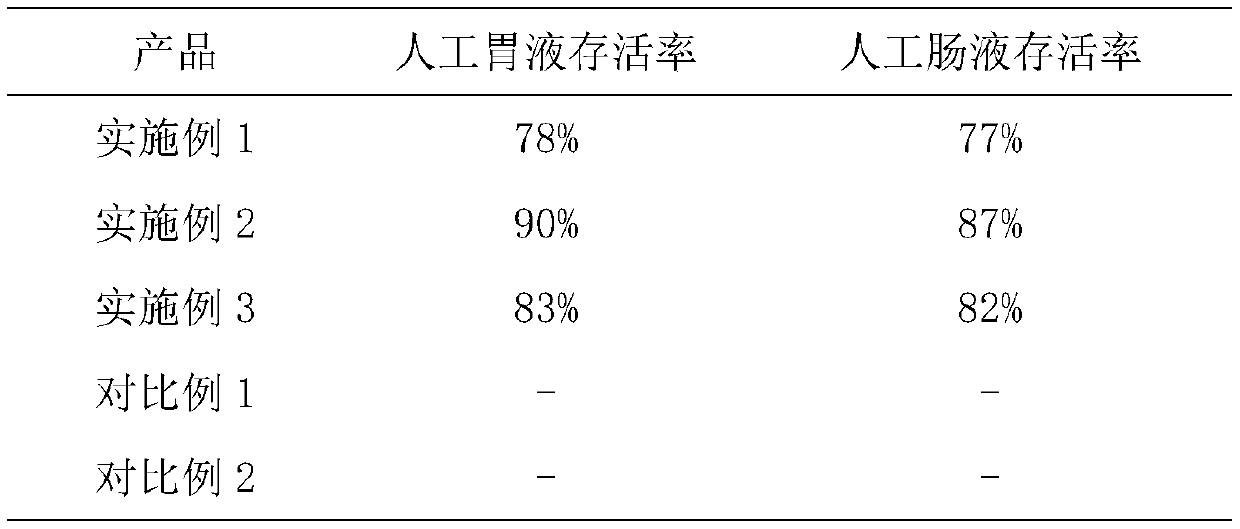
Image
Examples
Embodiment 1
[0026] Embodiment 1: Preparation of enteric-coated composite microcapsules
[0027] (1) Prepare 1 L of whey protein aqueous solution with a concentration of 20 g / L. After stirring continuously for 2 hours, centrifuge at 6000 rpm for 10 minutes, and keep the supernatant for later use.
[0028] (2) Prepare 1 L of gelatin aqueous solution with a concentration of 40 g / L, heat and stir until completely dissolved, cool to room temperature, and set aside.
[0029] (3) Bacillus subtilis is carried out strain activation, and after subculture twice, the concentration of Bacillus subtilis is about 10 9 CFU / mL, centrifuge 1L of Bacillus subtilis fermentation broth at 6000rpm for 10min, collect the bacterial sediment, disperse the bacterial sediment of Bacillus subtilis into the supernatant of whey protein, and slowly add gelatin aqueous solution , to mix the suspension.
[0030] (4) Spray-dry the suspension while stirring, the inlet air temperature is 105°C, the outlet air temperature i...
Embodiment 2
[0031] Embodiment 2: Preparation of enteric-coated composite microcapsules
[0032] (1) Prepare 2L of sodium caseinate aqueous solution with a concentration of 40g / L. After stirring continuously for 4h, centrifuge at 10000rpm for 10min, and keep the supernatant for later use.
[0033] (2) Prepare 2L of pectin aqueous solution with a concentration of 40g / L, stir until completely dissolved, and set aside.
[0034] (3) Lactobacillus plantarum was activated for strains. After two passages, the concentration of Lactobacillus plantarum was about 10 9 CFU / mL, centrifuge 4L of Lactobacillus plantarum fermentation broth at 6000rpm for 10min, collect the bacterial sediment, disperse the bacterial sediment of Lactobacillus plantarum into the supernatant of sodium caseinate, and slowly add pectin aqueous solution , to mix the suspension.
[0035] (4) Spray-dry the suspension while stirring, the inlet air temperature is 100°C, the outlet air temperature is 80°C, and the feed flow rate is...
Embodiment 3
[0036] Embodiment 3: Preparation of enteric-coated composite microcapsules
[0037] (1) Prepare 1 L of soybean protein aqueous solution with a concentration of 40 g / L, continue stirring for 2 hours, centrifuge at 6000 rpm for 10 minutes, and keep the supernatant for later use.
[0038] (2) Prepare 1 L of collagen aqueous solution with a concentration of 40 g / L, stir until completely dissolved, and set aside.
[0039] (3) Bacillus subtilis is carried out strain activation, and after subculture twice, the concentration of Bacillus subtilis is about 10 9 CFU / mL, centrifuge 1L of Bacillus subtilis fermentation broth at 6000rpm for 10min, collect the bacterial sediment, disperse the bacterial sediment of Bacillus subtilis into the supernatant of soybean protein, and slowly add it to the aqueous collagen solution , to mix the suspension.
[0040](4) Spray-dry the suspension while stirring, the inlet air temperature is 105°C, the outlet air temperature is 85°C, and the feed flow ra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com