Synthesis method and application of indole derivative capable of efficiently degrading perfluorinated compounds
A technology of indole derivatives and perfluorinated compounds, which is applied in the field of synthesis of indole derivatives, can solve the problems of insufficient utilization of hydrated electrons, reduced operability, and large dosage of pharmaceuticals, achieving high utilization, Improvement of utilization rate and effect of high utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Chemical synthesis of a novel indole derivative:
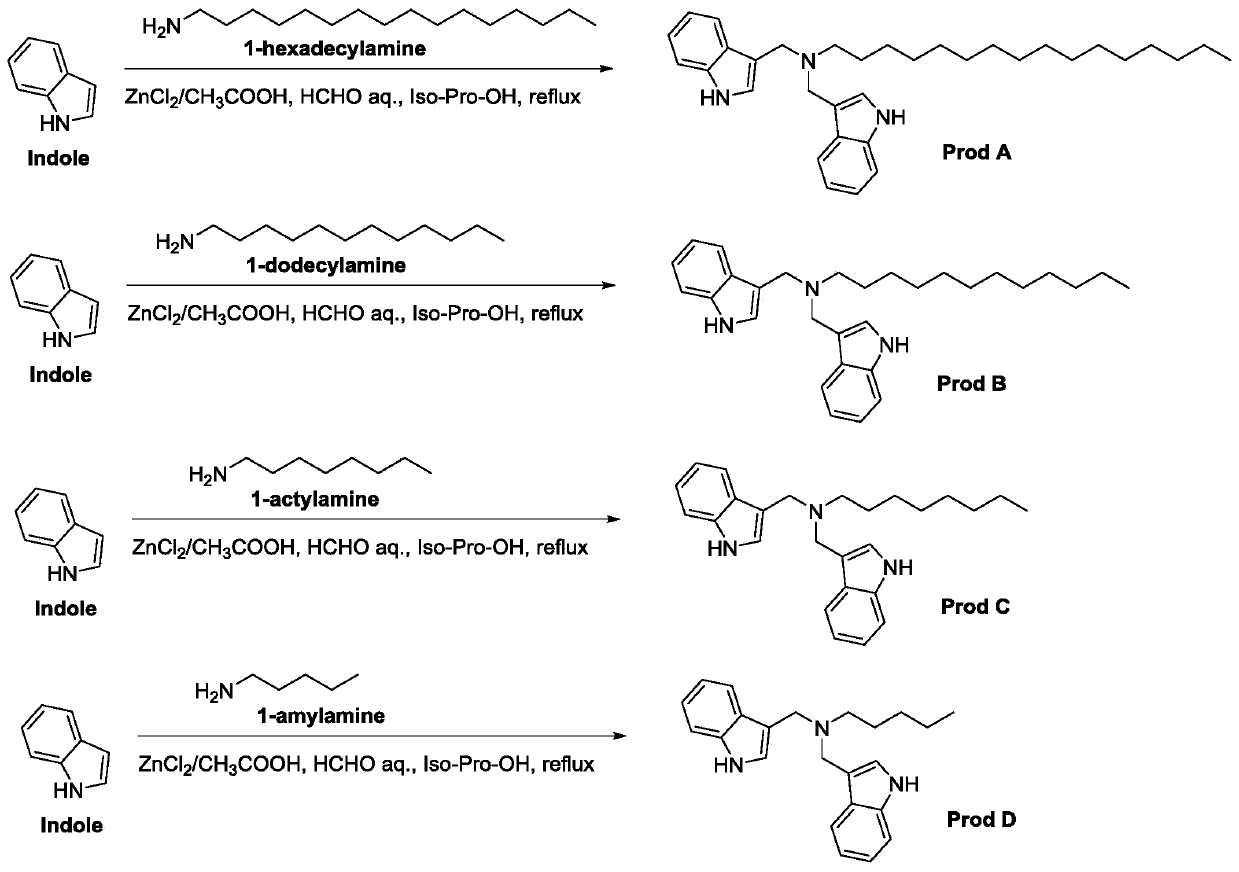
[0029] 5g of indole, 5.2g of n-hexadecylamine and 3.3mL of 35% formaldehyde solution were dissolved in 50mL of ethanol solution, and 1.2g of ZnCl was added 2 as a catalyst reaction. The reaction was refluxed at 55°C for 10 hours. The reacted product was filtered, evaporated to dryness to 10mL, separated and purified through a silica gel column (200-300 mesh), and finally obtained 3g of product A ( figure 1 ), the yield is 60%.
Embodiment 2
[0031] Chemical synthesis of a novel indole derivative:
[0032] (1) adopt indole, n-hexadecylamine, formaldehyde and Virahol identical with embodiment 1, take 5.1g glacial acetic acid as catalyst reaction, through same preparation and purification method, finally obtain 9.2g product A, synthetic The yield was 87%.
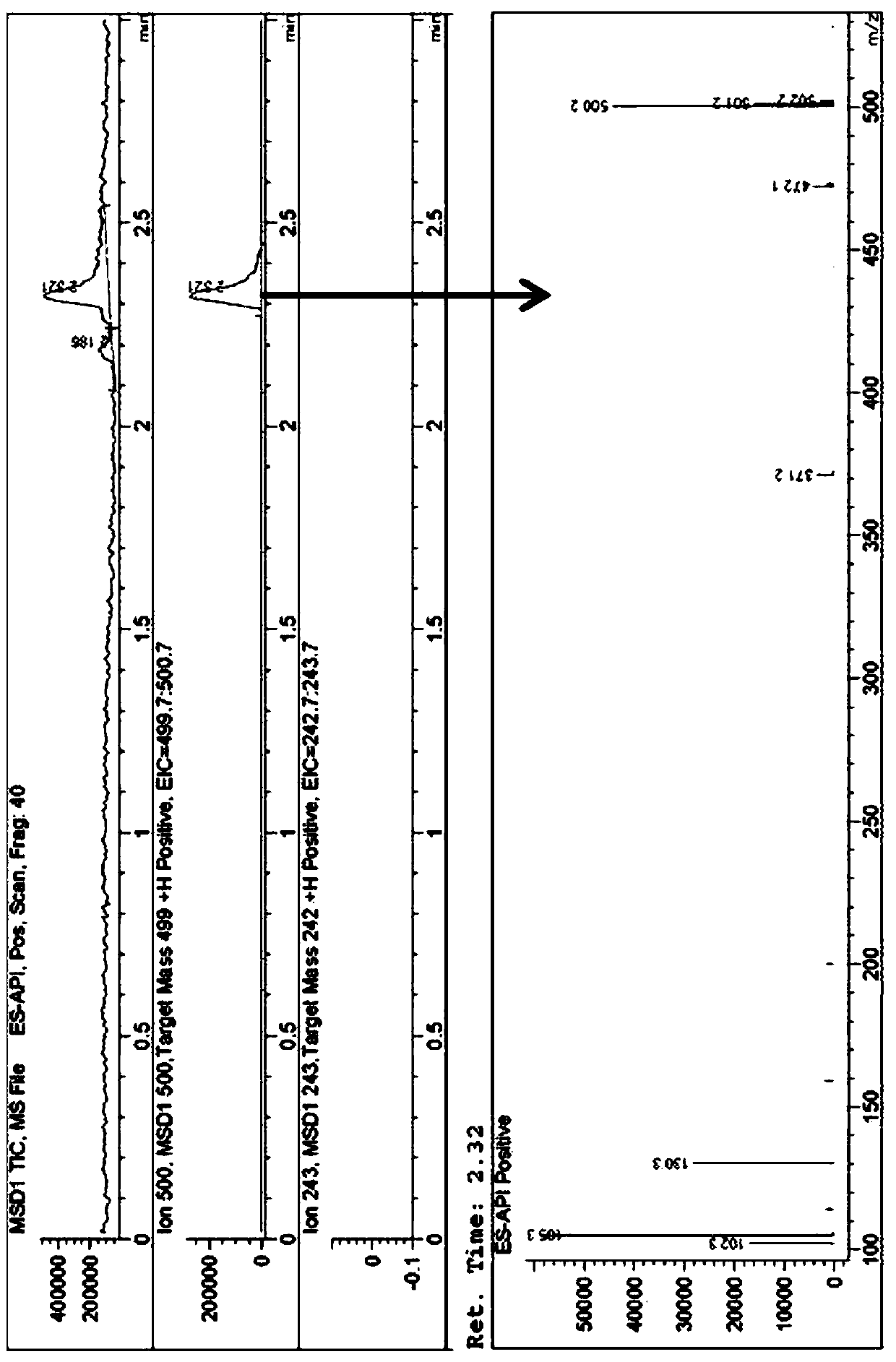
[0033] (2) The obtained product A was dissolved in isopropanol at a concentration of 10 mg / L. Agilent 1200 liquid chromatograph was used in series with Agilent 6120 mass spectrometer detector and electrospray ionization source was used to determine the purity of product A. Use Waters X-BrigdeShield C18 chromatographic column (50mm*4.6mm*3.5um) to separate, use water (containing 0.5% trifluoroacetic acid) and acetonitrile (containing 0.5% trifluoroacetic acid) as the mobile phase for gradient elution, and the flow rate is 2mL / min, the organic phase increased from 5% to 100% within 1.6min and kept for 1.4min. The purity of product A finally obtained was >88%. C...
Embodiment 3
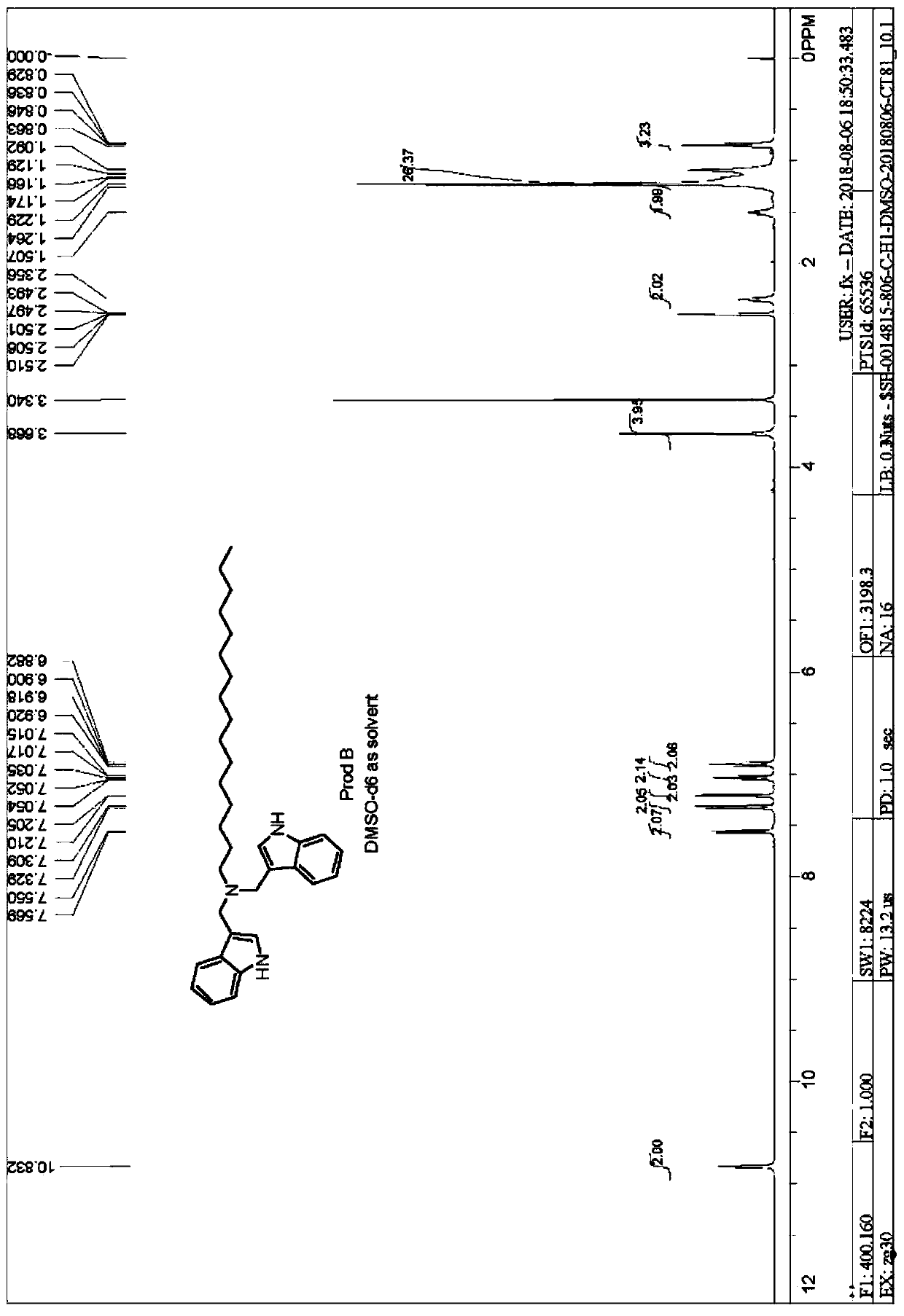
[0036]This synthetic method is also suitable for synthesizing derivatives with different alkyl chain lengths, and the n-hexadecylamine of Example 2 is replaced with other carbon chain lengths of alkylamines, including n-dodecylamine (H 2 N-C 12 h 25 ), n-octylamine (H 2 N-C 8 h 17 ), n-pentylamine (H 2 N-C 5 h 11 ), with glacial acetic acid as catalyst reaction, through the same preparation and purification method, synthesize product B respectively, product C, product D ( figure 1 ), the synthetic yields were all over 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com