Echinococcus multilocularis leucine aminopeptidase subunit vaccine LAP, preparation method and application thereof
A leucine amide peptidase subunit and multilocular hydatid technology is applied in the field of application and preparation of multilocular hydatid subunit vaccine leucine amide peptidase LAP, and can solve the risk of recurrence, poor patient compliance and side effects. It can prevent mice from infecting Echinococcus multilocularis, with high safety and stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Construction of recombinant expression vector pCzn1-LAP (containing fusion gene LAP)
[0044] The amino acid sequence of LAP was transformed into the corresponding nucleotide sequence according to the principle of E. coli codon preference, and the method based on PAS (PCR-based Accurate Synthesis) was adopted to design the full-length splicing primer, and the protection was designed at both ends of the primer. The sex base synthesis gene LAP is connected to the expression vector pCzn1 through the cloning sites Nde I and Xba I.
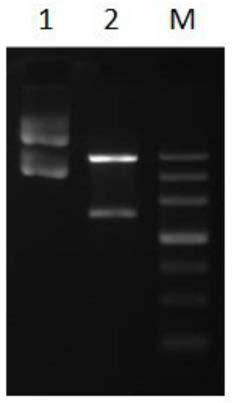
[0045] Results: The recombinant plasmid pCzn1-LAP to be tested was double digested with Nde I and Xba I, reacted at 37°C for 2 hours, and detected by 1% agarose gel electrophoresis. It was found that the double digested DNA fragment was about 1700 bp, and the fusion gene LAP The theoretical size of is the same, such as ( figure 1 ) Shown. The vector construction map of the recombinant expression vector pCzn1-LAP is as follows ( figure 2 ...
Embodiment 2
[0046] Example 2: Prokaryotic expression of multi-epitope peptide fusion protein LAP
[0047] The correct recombinant expression plasmid pCzn1-LAP was transformed into the Escherichia coli Arctic Express strain. On the pre-prepared LB plate containing 50μg / mL Amp, inoculate the genetically engineered strain pCzn1-LAP / ArcticExpress, placed upside down in a 37℃ incubator, after overnight culture, pick a single colony and inoculate it with 50μg / mL Cultivate overnight in Amp's LB medium at 37°C and 220 rpm. Inoculate the recombinant bacteria in 50μg / mL Amp LB medium with 2% inoculum, at 37°C, 220rpm, culture until the OD600 of the bacteria is 0.6-0.8 (about 2h), add IPTG to make the final concentration 1mmol / L, The expression was induced at 37°C and 220 rpm for 4 hours, and the carrier strain pCzn1-LAP / Arctic Express without IPTG induction was used as a negative control.
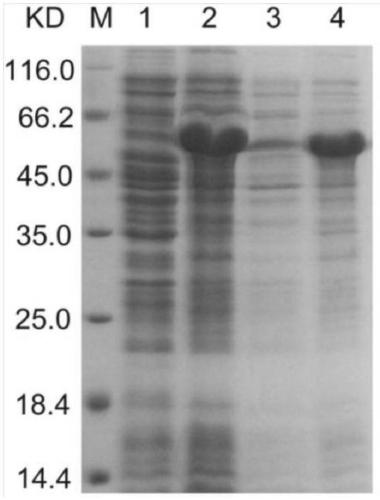
[0048] Results: Compared with the control strain, the genetically engineered recombinant strain pCzn1-LAP / Arctic...
Embodiment 4
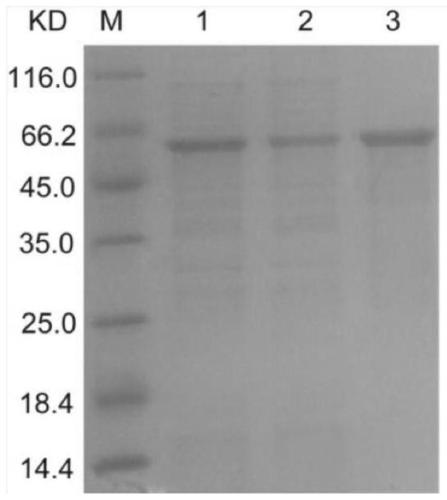
[0049] Example 4: Purification of multi-epitope peptide fusion protein LAP
[0050] (1) Denaturation of inclusion body protein
[0051] Resuspend the bacterial pellet in 20ml lysis buffer (20mM Tris-HCl containing 1mM PMSF and bacteria protease inhibitor cocktail, pH 8.0), ultrasonically disrupt (power 400W, work 4sec, interval 8sec, total 20min); ultrasonically disrupt the cell lysate Centrifuge at 10000g for 20min at 4℃, and collect the precipitate; wash the inclusion bodies 3 times with the inclusion body washing solution (20mM Tris, 1mM EDTA, 2M urea, 1M NaCl, 1% Triton X-100, pH8.0); Tris, 5mM DTT, 8M urea (pH8.0), dissolve the inclusion bodies according to a certain proportion, and place overnight at 4℃; centrifuge at 15000rpm for 15min at room temperature; add the above solution dropwise to 20mM Tris-HCl 5mM EDTA Buffer PH7.8, gradually Dilute in multiples with slow stirring, put the protein solution into a dialysis bag and dialyze in PBS pH7.4 solution overnight.
[0052] (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com