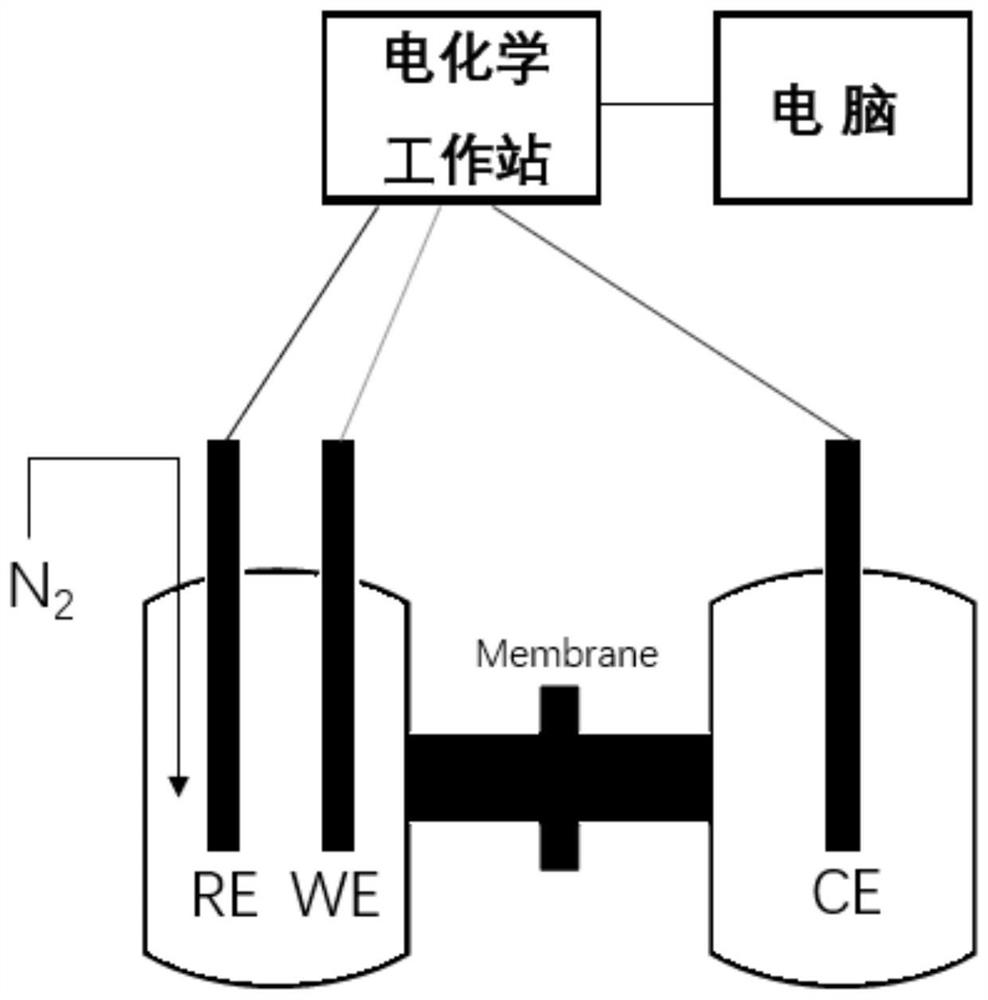
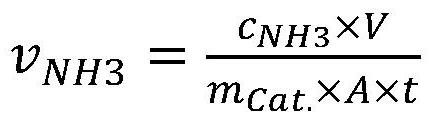Ammonia synthesis method for electrocatalytic reduction of nitrogen, and catalyst used in method
A catalyst and electrocatalysis technology, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, catalyst activation/preparation, etc., can solve the problems of large energy consumption, low ammonia production rate, and low yield, and achieve the preparation method Simple, wide source of raw materials, enhanced catalytic activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0039] Example one, the preparation of the carbon material (carbon material carrier) as carrier:
[0040] Mix 1g of high-purity carbon powder (purity ≥ 95%), 1g of sodium nitrate and 46mL of concentrated sulfuric acid (sulfuric acid solution with a mass concentration of 95-98%) in an ice bath (about 0°C), and add 6g of permanganic acid Potassium, then react at 30-40°C for 1h, continue to add 40mL of deionized water, and heat the mixture in a water bath at 90°C for 30-50min, after taking it out, add 100mL of deionized water to stop the reaction, and add 6mL of hydrogen peroxide solution (a hydrogen peroxide solution with a mass concentration of 30%, which acts to reduce residual oxidant potassium permanganate); finally, repeatedly wash with 3% (mass %) hydrochloric acid and deionized water until the pH of the washing solution is close to neutral. After adding about 20ml of water to the cleaned product, perform ultrasonic dispersion for 2 hours (ultrasonic dispersion: 25°C, powe...
Embodiment 1
[0042] Embodiment 1, preparation of V / G single-atom catalyst by photodeposition method:
[0043] Disperse 50mg of TiO in 30ml of mixed solvent of water:methanol=8:1(v / v) 2 and 50mg carbon material up to TiO 2 , The carbon material is uniformly dispersed to obtain a dispersion liquid;
[0044] Add about 2.39 mg of sodium metavanadate (containing 1 mg of vanadium) to the above dispersion, then put it under the light source and stir at room temperature for 5 hours, then freeze it in the refrigerator (-20°C) for 2 hours until the solution solidifies into a solid, and finally vacuum freeze-dry After 24 hours, 101.0 mg of a V / G single-atom catalyst with a mass fraction of 1.0% was obtained;
[0045] The light source is provided by a short-arc xenon lamp steady current power supply, the current is 10A, the power of the xenon lamp is 500W, and the wavelength range of the light source is 320-780nm.
Embodiment 2
[0046] Embodiment 2, photodeposition method prepares Nb / G single-atom catalyst:
[0047] Disperse 50 mg of TiO in 30 ml of a mixed solvent of water: isopropanol = 10:1 (v / v) 2 and 50mg carbon material up to TiO 2 , The carbon material is uniformly dispersed to obtain a dispersion liquid;
[0048] Add about 5.79 mg of niobium oxalate (containing 1 mg of Nb) to the above dispersion, then place it under a light source, irradiate at room temperature and stir for 3 hours, then freeze in the refrigerator for 2 hours until the solution solidifies into a solid, and finally vacuum freeze-dry for 24 hours to obtain the mass fraction 101.0 mg of 1.0% Nb / G single-atom catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com