Preparation method of valnemulin hydrochloride intermediate as veterinary drug bulk drug
A technology of warnemulin hydrochloride and raw materials, which is applied in the field of preparation of warnemulin hydrochloride intermediates of veterinary drug raw materials, can solve the problems of reduced yield, complicated preparation process, long reaction time, etc., and achieve mild reaction conditions, Simple purification process and low solvent toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation method of a veterinary raw material drug Vonimulin hydrochloride intermediate
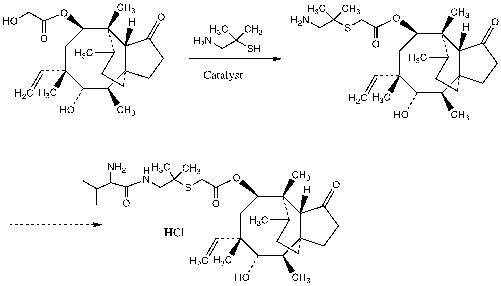
[0039] The veterinary raw material drug intermediate is a pleuromutilin dimethylcysteamine substitute, and its preparation method includes the following steps:
[0040] Take 11.36 g (0.03 mol) of pleuromutilin and dissolve it in 200 mL of toluene. After it is dissolved, add 4.25 g (0.03 mol) of dimethylcysteamine hydrochloride, and then add 2.4 g (0.06 mol) of sodium hydroxide. ) And solid catalyst Al 2 O 3 on silica(0.6%)(4g, 0.18mmol), keep the temperature at 80-85°C and stir the reaction for 10 h; after the reaction is complete, add 10 g of boric acid, stir and react for 30 minutes, then filter and discard the filter residue; the filtrate is concentrated to dryness , Recrystallized with methyl tert-butyl ether to obtain 11.9 g of pleuromutilin dimethylcysteamine substitute, which is the same as the high-pressure liquid phase retention time of the intermediate synthesized...
Embodiment 2
[0042] Example 2 Preparation method of a veterinary raw material drug Vonimulin hydrochloride intermediate
[0043] The veterinary raw material drug intermediate is a pleuromutilin dimethylcysteamine substitute, and its preparation method includes the following steps:
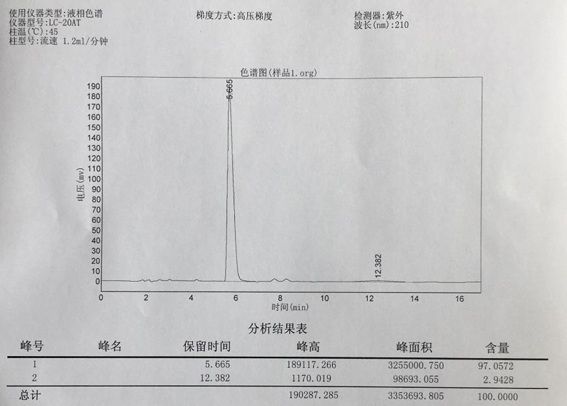
[0044] Take 11.36 g (0.03 mol) of pleuromutilin and add it to 200 mL of methyl tert-butyl ether, then add 4.25 g (0.03 mol) of dimethylcysteamine hydrochloride, and then add 1.2 g (0.03 mol) of sodium hydroxide mol) and aluminum tungstophosphate AlPW 12 O 40 (6 g, 0.021 mol), the reaction was stirred at room temperature for 2 h; after the reaction was completed, 10 g of boric acid was added and reacted for 30 minutes; filtered, the filter residue was washed with methyl tert-butyl ether and discarded, the filtrate was concentrated in vacuo to precipitate a solid, filtered , Dried to obtain 10.5 g of pleuromutilin dimethylcysteamine substitute, with a yield of 75% and a purity of 97.10%.
Embodiment 3
[0045] Example 3 Preparation method of a veterinary raw material drug Vonimulin hydrochloride intermediate
[0046] Under the protection of nitrogen, add 100 mL of methyl tert-butyl ether, 23.5 g (0.062 mol) of pleuromutilin, 9.83 g (0.0694 mol) of dimethylcysteamine hydrochloride, and hexachloro ring into a 2L three-necked flask. Triphosphazene (TAPC) (2.1g, 0.0062mol), 1.5 g of benzyltributylammonium chloride, heated to 50°C and stirred for 1 hour, after removing the solvent in vacuum, add 100 mL H2O and 70 mL 1mol / L NaOH solution Into the reaction mixture, cool to 10°C, stir for 15min and filter, wash the filter cake with water and cold methyl tert-butyl ether (30ml each), and dry at 55°C to obtain pleuromutilin dimethylcysteamine substitution The product is 27.4g, the yield is 95%, and the purity is 97.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com