Organic compound, organic electroluminescent material and application thereof
An organic compound, selected technology, applied in the field of organic compounds and organic electroluminescent materials, can solve problems such as inability to meet application requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
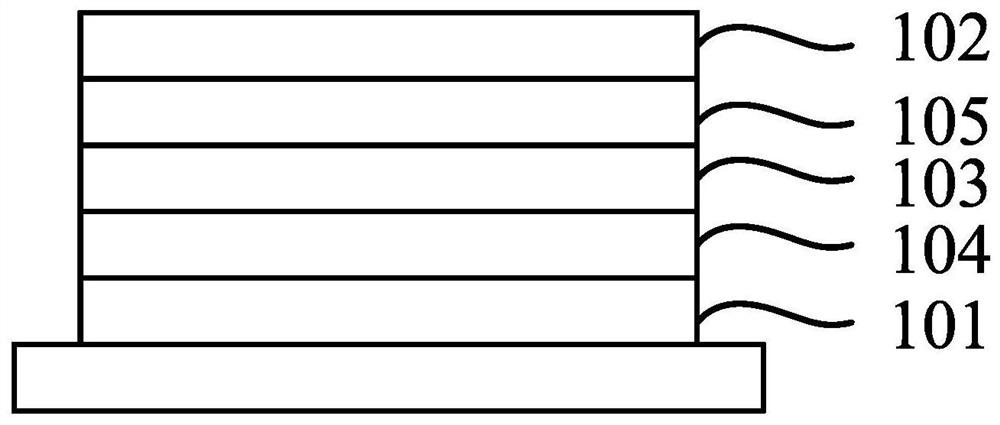
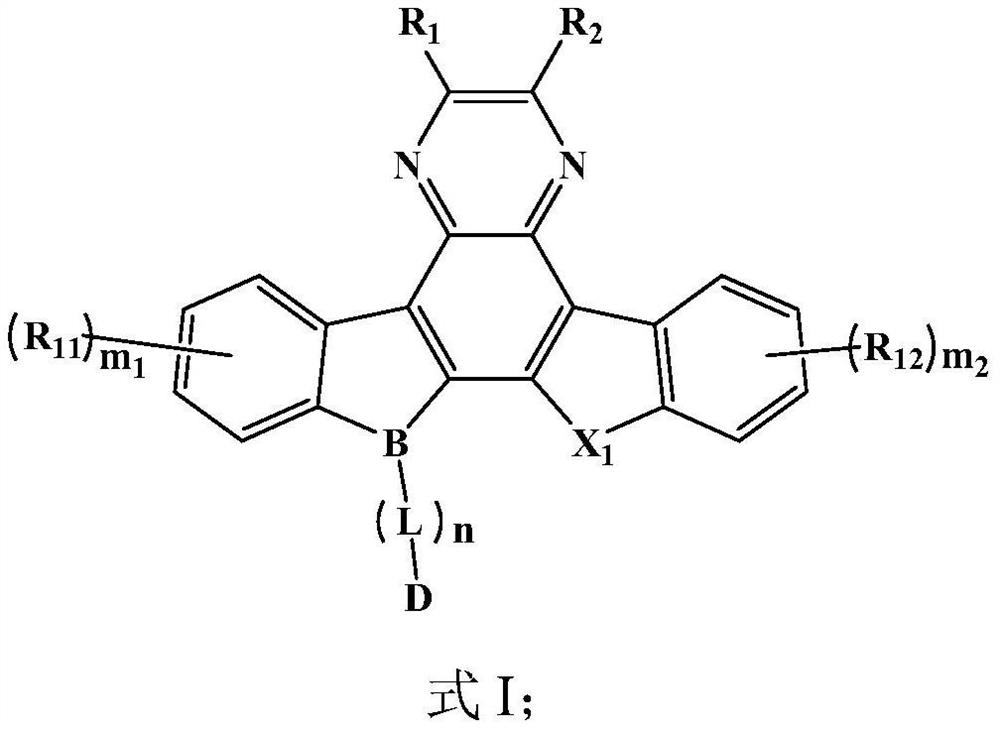
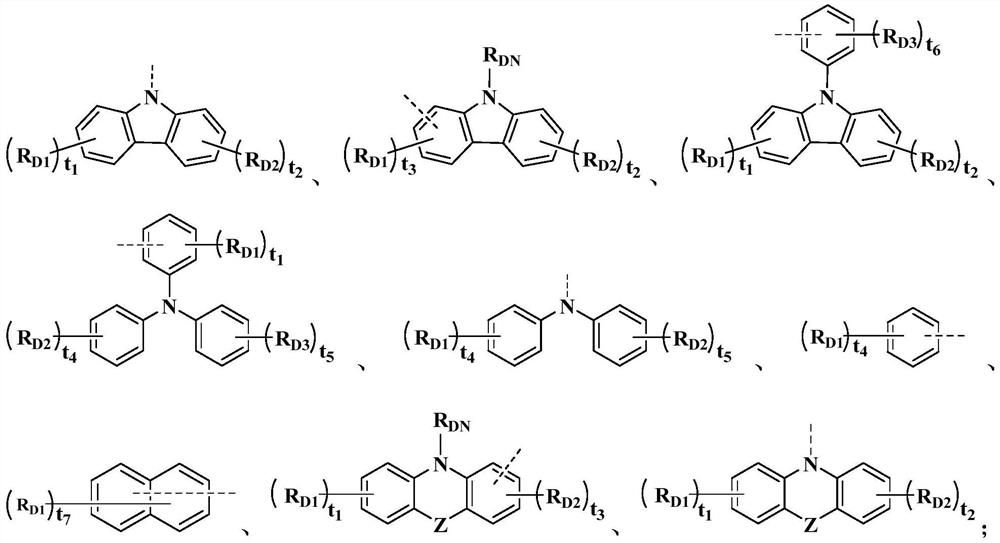
Image
Examples
Embodiment 1
[0106]This embodiment provides an organic compound with the following structure:
[0107]
[0108] The preparation method of this organic compound H008 comprises the steps:
[0109] (1)
[0110] In a 250 mL round bottom flask, compound A (15 mmol) and potassium acetate (KOAc, 40 mmol) were mixed with dry 1,4-dioxane (60 mL), bis(triphenylphosphine)palladium chloride Pd(PPh 3 ) 2 Cl 2 (0.4mmol) and diboronic acid pinacol ester (25mmol) were mixed, stirred at 90°C under nitrogen atmosphere for 48h. The resulting intermediate was cooled to room temperature, added to water, and filtered through a pad of celite. The filtrate was extracted with dichloromethane, washed with water, and dried over anhydrous magnesium sulfate. After filtration and evaporation, the crude product was purified by silica gel column chromatography. The product yielded intermediate H008-1.
[0111] (2)
[0112] In a 250mL round bottom flask, the intermediate product H008-1 (12mmol), 1-chloro-2-br...
Embodiment 2
[0119] This embodiment provides an organic compound with the following structure:
[0120]
[0121] The preparation method of this organic compound H009 comprises the steps:
[0122]
[0123] Add the intermediate product H008-2 (10mmol, the same preparation method as in Example 1) to a 250mL round bottom flask, replace the air with nitrogen three times, add tetrahydrofuran (50mL), place under a dry ice-ethanol bath and stir for half an hour, add t -BuLi(30mmol) was stirred for half an hour, then added (MeO) 3 B (25mmol), continue stirring for 1h. Take another single-necked bottle, add compound C (22mmol), tetrahydrofuran (50mL), place it in an ice-water bath and stir for 1h, add i-PrMgCl·LiCl tetrahydrofuran solution (80mmol) and stir for 1h, (MeO) 3 A solution of B in THF was added to the reaction. Stir for 2 h, add distilled water, concentrate, and use dichloromethane to extract the organic matter three times. The organic phases were combined and concentrated, and ...
Embodiment 3
[0128] This embodiment provides an organic compound with the following structure:
[0129]
[0130] The preparation method of this organic compound H020 comprises the steps:
[0131] (1)
[0132] In a 250 mL round bottom flask, Compound D (15 mmol) and KOAc (40 mmol) were mixed with dry 1,4-dioxane (70 mL), Pd(PPh 3 ) 2 Cl 2 (0.4mmol) and diboronic acid pinacol ester (25mmol) were mixed, stirred at 90°C under nitrogen atmosphere for 48h. The resulting intermediate was cooled to room temperature, added to water, and filtered through a pad of celite. The filtrate was extracted with dichloromethane, washed with water, and dried over anhydrous magnesium sulfate. After filtration and evaporation, the crude product was purified by silica gel column chromatography. The product yielded intermediate H020-1.
[0133] (2)
[0134] In a 250mL round bottom flask, the intermediate product H020-1 (12mmol), 1-chloro-2-bromobenzene (10mmol) and Pd(PPh 3 ) 4 (0.3mmol) was added t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com