Non-invasive near-infrared light-controlled nano material for treating diabetes mellitus
A technology of fluorescent nanomaterials and inorganic nanomaterials, which is applied in the field of diabetes medical research, can solve problems such as the unknown effectiveness of diabetes treatment, and achieve the effect of getting rid of the shackles of optical fibers, avoiding influence, and avoiding side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
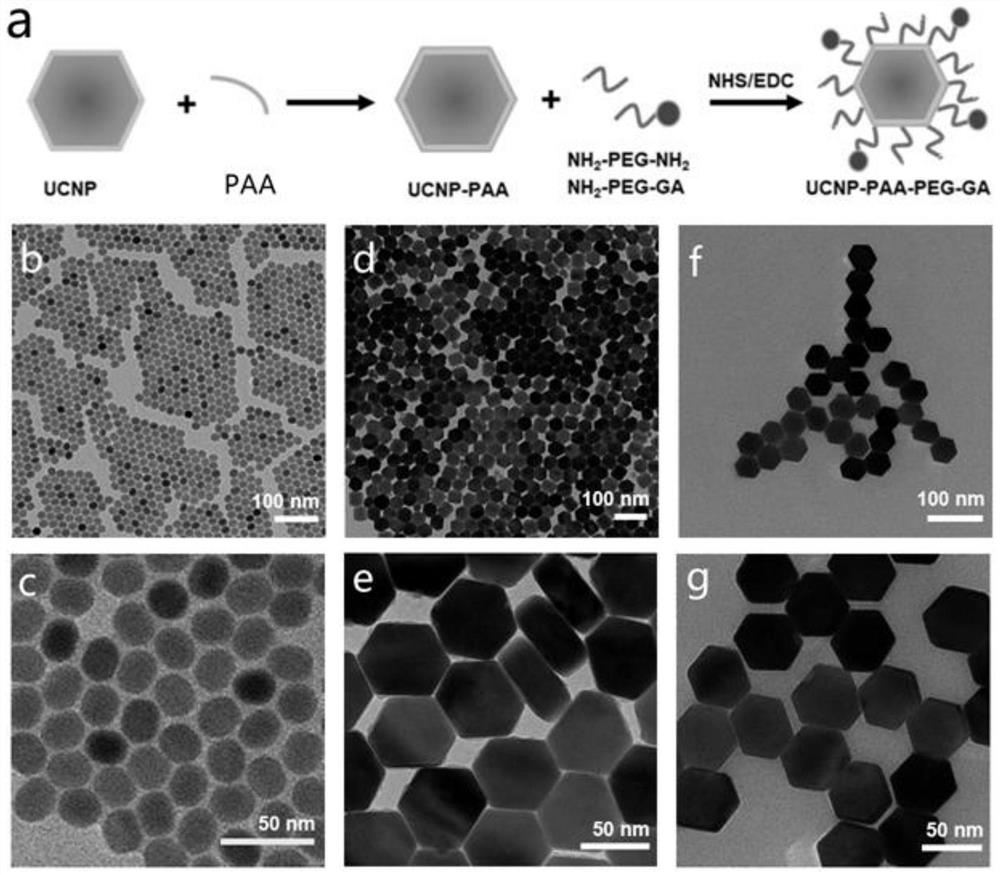
[0055] Embodiment 1: the synthesis of UCNP-PEG-GA
[0056] 1. NaYF with core-shell structure 4 :Yb / Tm@NaYF 4 Synthesis of upconverting nanoparticles (UCNPs):
[0057] NaYF with core-shell structure 4 :Yb / Tm@NaYF 4 Upconverting nanoparticles were synthesized by a thermal solvent method.
[0058] First synthesize nuclear NaYF 4 :Yb / Tm: Weigh 0.695mmol YCl on a weighing balance 3 (135.78 mg), 0.30 mmol YbCl 3 (83.82mg) and 0.005mmol TmCl 3 (1.38 mg), was added to a 50 mL three-necked flask, followed by 12 mL of oleic acid and 15 mL of octadecene. After fixing the three-necked flask on the heating platform, pass nitrogen gas into the reaction device for 5 minutes to remove the air, then start to heat up, and keep the reaction system at 160°C for 0.5 hours under magnetic stirring to dissolve the reactants and remove the reaction system. excess oxygen and moisture. Turn off the heating, and wait for the reaction system to cool down to room temperature. At this time, the pr...
Embodiment 2
[0067] Embodiment 2: cell model and related research
[0068] HepG2 cells were purchased from ATCC, and HUVEC cells were a gift from Tang Zhongying Hematology Research Center. Cells were cultured in DMEM medium containing 10% FBS and 25 mM glucose at 37°C with 5% CO 2 in a humid environment.
[0069] In order to obtain the HepG2 cell model of insulin resistance, HepG2 cells were induced and cultured for 18 hours in DMEM low-glucose medium containing 18mM glucosamine (glucosamine, GlcN) and 5mM glucose, to obtain the HepG2 cell model of insulin resistance. Plasmid transfection was completed by jetPRIME (Polyplus) reagent. When the cells grew to 50% density, the plasmid CIBN-CAAX and mCherry-CRY2-iSH were added to the transfection buffer at a ratio of 1:1.2, vortexed for 5 seconds and then added to the transfection buffer. Transfection reagent (1 μg plasmid corresponds to 50 μL transfection buffer and 1 μL transfection reagent), put it into the cell culture medium after standi...
Embodiment 3
[0077] Example 3: HepG2 cell insulin resistance model and related research
[0078] Glucosamine (GlcN)-induced insulin resistance model of HepG2 cells was used to evaluate the effect of UMO in vitro. Figure 10 a-d are the results of cell immunofluorescence staining experiments, where Figure 10 a and b are the results of immunofluorescence staining of cells in the normal control group and the GlcN-induced group under the condition of NIR light off, respectively; Figure 10 c and d are the results of immunofluorescence staining of cells in the normal control group and GlcN-induced group under the condition of NIR light on, respectively. In the figure, different colors are used to represent the fluorescent signals of mCherry, DAPI, and p-AKT. It can be seen from the cell immunofluorescence staining experiment ( Figure 10 ), in the normal group ( Figure 10 c) and GlcN treatment group ( Figure 10 d) The fluorescence signal intensity of p-AKT after being irradiated by NIR wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com