Porphyrin-based temperature-responsive self-healing hydrogel, preparation method, use method and application thereof
A temperature-responsive, self-healing technology, applied in the field of medical polymer materials and functional polymer materials, to achieve the effect of simple preparation method, good injectability, and good mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
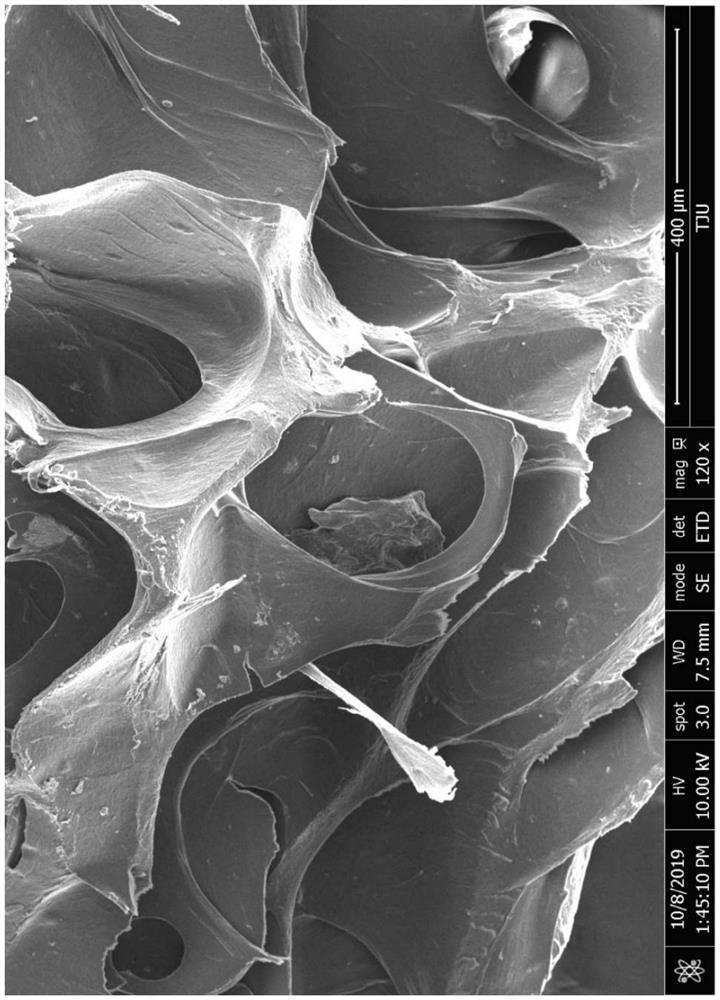
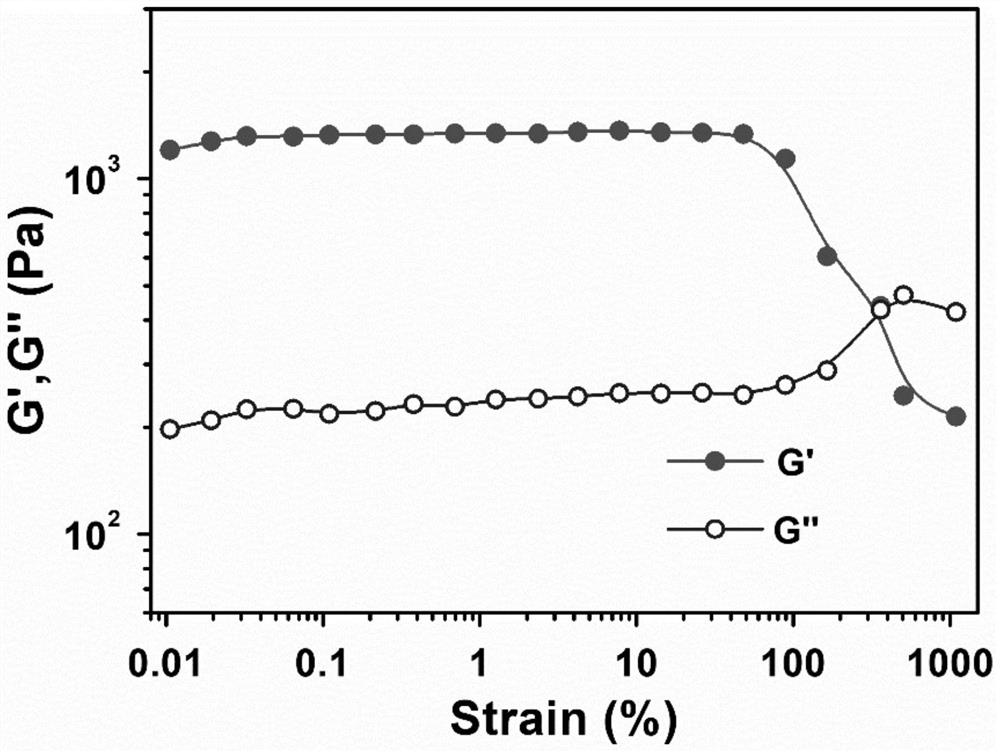
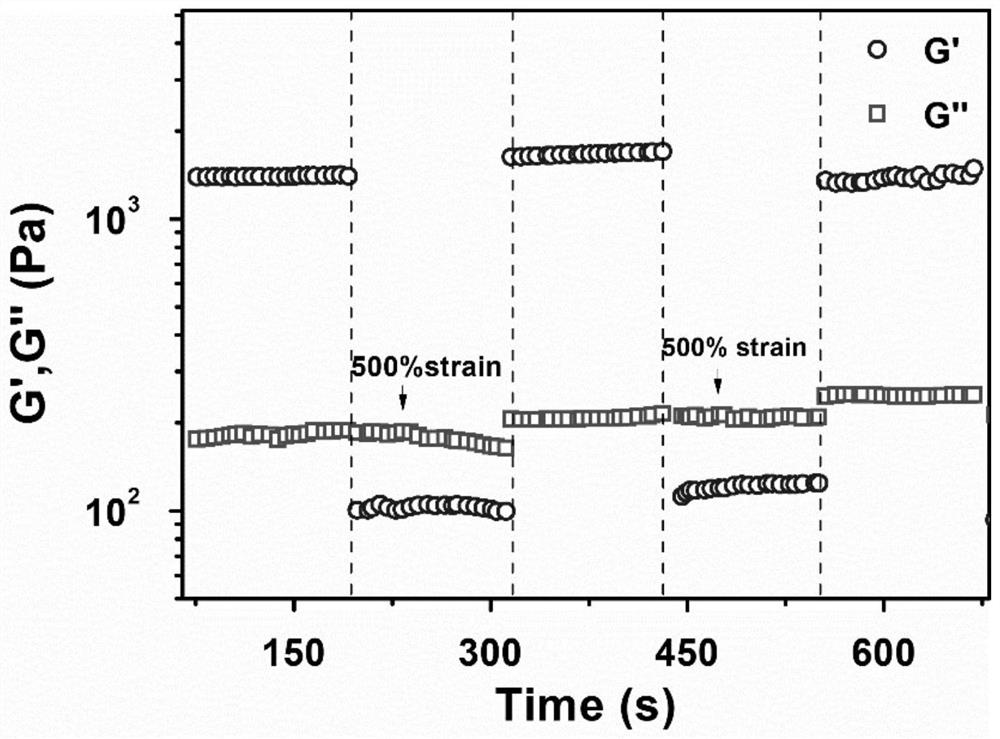
Image
Examples
Embodiment 1
[0041] Dissolve 0.1 g of 5,10,15,20-tetra-p-hydroxyphenylporphyrin (THPP) in dichloromethane; add 0.27 g of 2-bromoisobutyryl bromide and 0.12 g of triethylamine, and react at 0°C for 48 hours , remove residual 2-bromoisobutyryl bromide, triethylamine and dichloromethane to obtain THPP(-Br) 4 Initiator (Product 1).
[0042] Dissolve 0.08 g of product 1 in N,N-dimethylformamide, add 4.22 g of 2-methyl-2-acrylic acid-2-(2-methoxyethoxy)ethyl ester, 0.59 g of oligo(ethyl) Diol) methyl ether methacrylate, 0.16 g of hydroxyethyl methacrylate; under the action of the catalyst cuprous bromide / pentamethyldiethylenetriamine, the system reacts under the protection of inert gas such as argon or nitrogen, The reaction temperature was 60° C., and the reaction time was 6 hours. After removing the catalyst, it was precipitated in petroleum ether, filtered and dried to constant weight to obtain THPP(-MEO 2 MA-co-OEGMA-co-HEMA) 4 (Product 2).
Embodiment 2
[0046] Dissolve 0.1 g of 5,10,15,20-tetra-p-hydroxyphenylporphyrin (THPP) in chloroform; add 0.42 g of 2-bromoisobutyryl bromide and 0.21 g of triethylamine, and react at 3°C for 36 hours , remove residual 2-bromoisobutyryl bromide, triethylamine and chloroform to obtain THPP(-Br) 4 Initiator (Product 1).
[0047] Dissolve 0.08 g of product 1 in toluene, add 4.22 g of 2-methyl-2-acrylic acid-2-(2-methoxyethoxy)ethyl ester, 1.18 g of oligo(ethylene glycol) methyl ether methyl ester Acrylate, 0.30 g hydroxyethyl methacrylate; under the action of catalyst cuprous bromide / pentamethyldiethylenetriamine, the system reacts under the protection of inert gas such as argon or nitrogen, the reaction temperature is 80 °C, and the reaction time For 10 hours, after removing the catalyst, it was precipitated in n-hexane, filtered and dried to constant weight to obtain THPP(-MEO 2 MA-co-OEGMA-co-HEMA) 4 (Product 2).
[0048] 0.22 g of p-aldehyde benzoic acid was added to 1 g of product ...
Embodiment 3
[0050] Dissolve 0.1 g of 5,10,15,20-tetra-p-hydroxyphenylporphyrin (THPP) in tetrahydrofuran; add 0.40 g of 2-bromoisobutyryl bromide and 0.12 g of pyridine, and react at 5°C for 40 hours to remove residual 2 -bromoisobutyryl bromide and pyridine and tetrahydrofuran to give THPP(-Br) 4 Initiator (Product 1).
[0051] Dissolve 0.08 g of product 1 in N,N-dimethylformamide, add 4.22 g of 2-methyl-2-acrylic acid-2-(2-methoxyethoxy)ethyl ester, 1.0 g of oligo(ethoxy) diol) methyl ether methacrylate, 0.28 g of hydroxyethyl methacrylate; under the action of the catalyst cuprous chloride / pentamethyl diethylene triamine, the system reacts under the protection of inert gas such as argon or nitrogen, The reaction temperature was 80°C, and the reaction time was 12 hours. After removing the catalyst, it was precipitated in diethyl ether, filtered and dried to constant weight to obtain THPP(-MEO 2 MA-co-OEGMA-co-HEMA) 4 (Product 2).
[0052] Add 0.11 gram of p-aldehyde benzoic acid to 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com