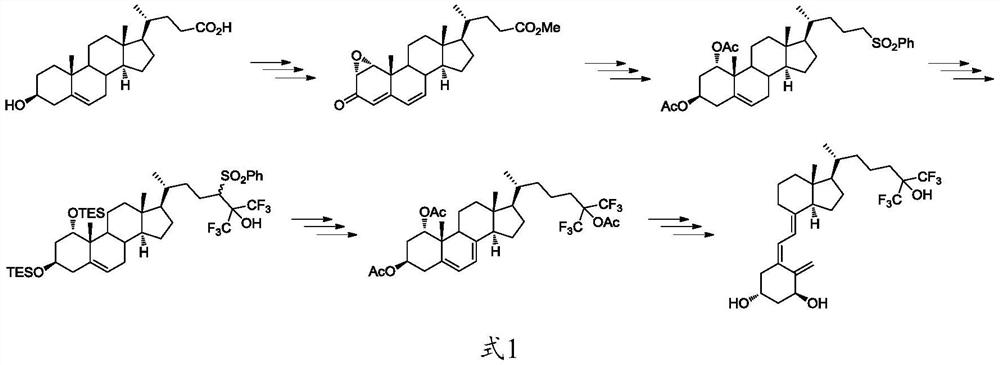
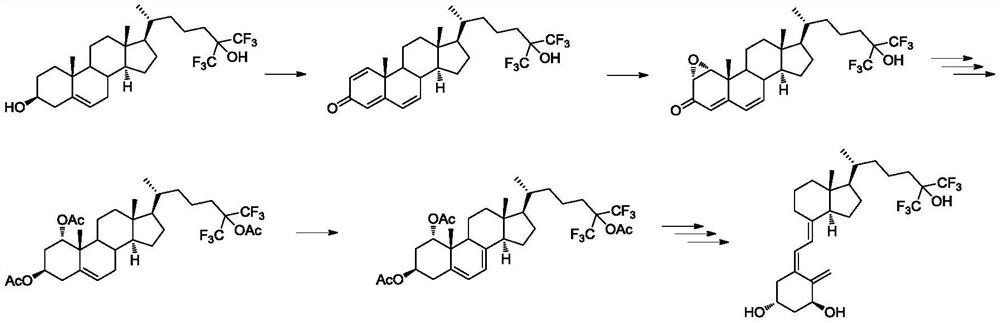
The preparation method and application of the intermediate for the preparation of flucalcidol
A technology for intermediates and calcidol, applied in the field of preparation of intermediates, can solve the problems of low safety, high cost, expensive raw materials and the like, and achieve the effects of simplifying reaction steps, high yield and reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Preparation of Compound 3
[0061] In a 2000ml there-necked flask, add compound 1 (30.0g, 258.4mmol, 0.861mol / L), n-hexane (300ml), cool the reaction to -60°C to -65°C, dropwise add lithium diisopropylamine (258.4ml, 258.4 mmol), keep the temperature reaction for 30min, slowly add the n-hexane saturated solution (260.0ml) of compound 2 dropwise, keep the temperature reaction for 1h, quench the reaction with saturated aqueous ammonium chloride solution, separate the organic phase, dry and concentrate to obtain 36.4g compound 3. The yield is 50.0%. Preparation of saturated hexafluoroacetone in n-hexane solution: drop the trihydrate of hexafluoroacetone into concentrated sulfuric acid, and absorb the generated gas with n-hexane (-20°C to -40°C) until saturation.
Embodiment 2
[0062] Example 2: Preparation of Compound 4
[0063] Under argon protection, compound 3 (35.0g, 124.1mmol) was added in a 500mL there-necked flask, then dichloromethane (300.0ml) was added, diisopropylethylamine (48.1g, 372.3mmol) was added, and the temperature was lowered to 0 ℃ was added methoxychloromethane (19.8g, 248.2mmol), the temperature was naturally raised to room temperature, stirred for 36 hours, the reaction solution was added to the aqueous sodium bicarbonate solution, extracted with ethyl acetate, dried and concentrated to obtain the crude product, which was subjected to silica gel column chromatography 28.3 g of compound 4 were obtained with a yield of 70.0%.
Embodiment 3
[0064] Example 3: Preparation of Compound 5
[0065] Compound 4 (28.0g, 85.8mmol) was added to a 500mL there-necked flask, tetrahydrofuran (280.0ml) was added, cooled to -5°C to 0°C, and lithium aluminum hydride (3.3g, 85.8mmol) was added in batches while maintaining the temperature. The temperature was reacted for 1 hour, the reaction was quenched by adding sodium hydroxide aqueous solution, extracted with ethyl acetate, dried and concentrated to obtain a crude product, and 15.8 g of compound 5 was obtained by silica gel column chromatography with a yield of 72.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com