A kind of preparation method of high-purity butylphthalide
A high-purity technology of butylphthalide, which is applied in the field of preparation of butylphthalide, can solve problems such as difficulty in column chromatography separation and purification, long reflux time, and insufficient product purity, and achieves a mild and controllable preparation process and high impurity content in the product. Low, the effect of improving safety performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
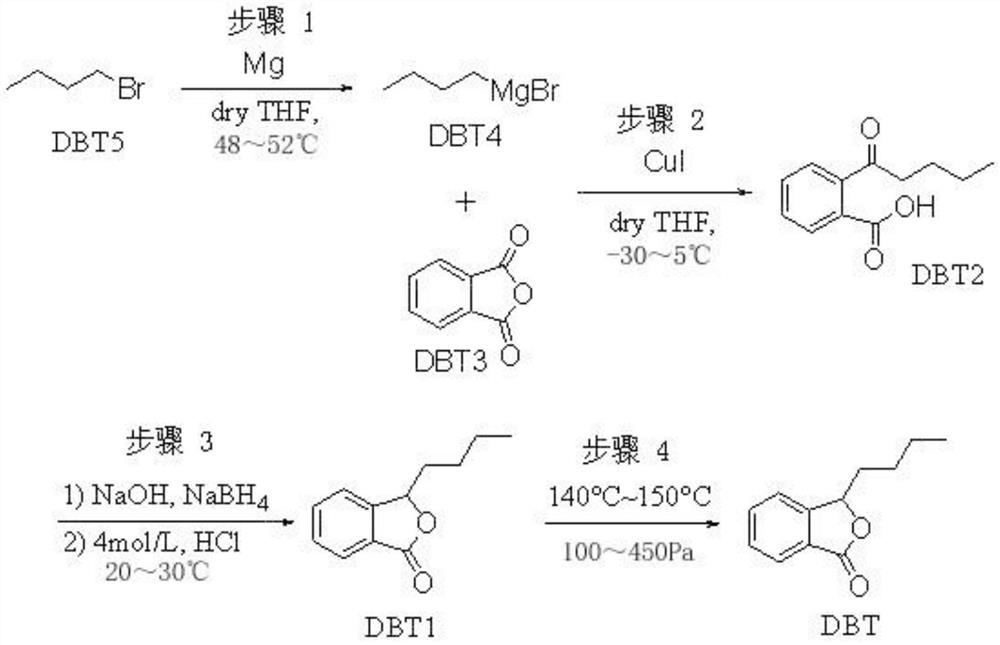
[0034] The invention provides a kind of preparation method of high-purity butylphthalide, and reaction process is as follows:
[0035]
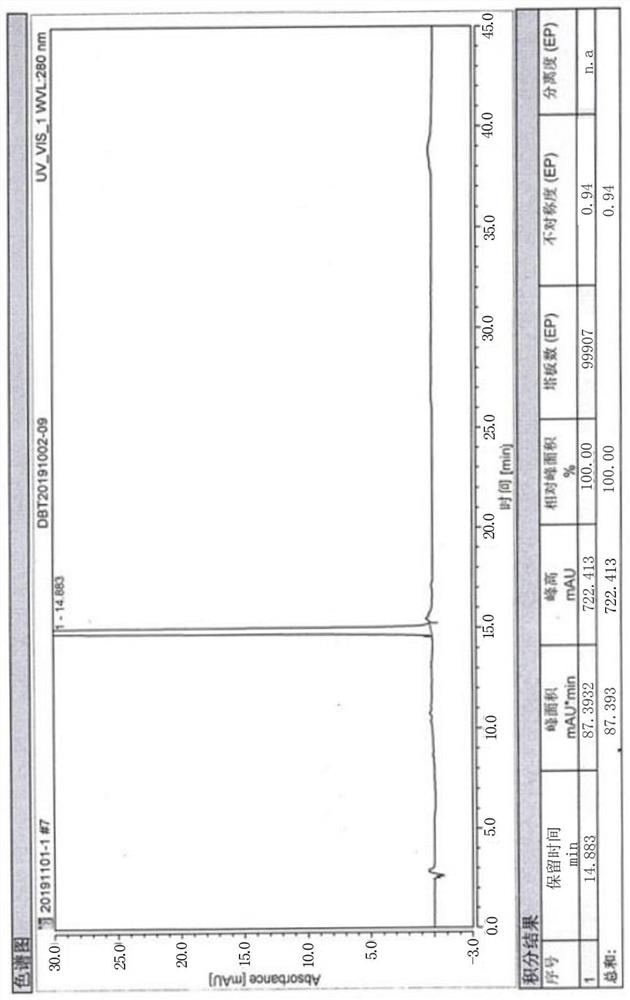
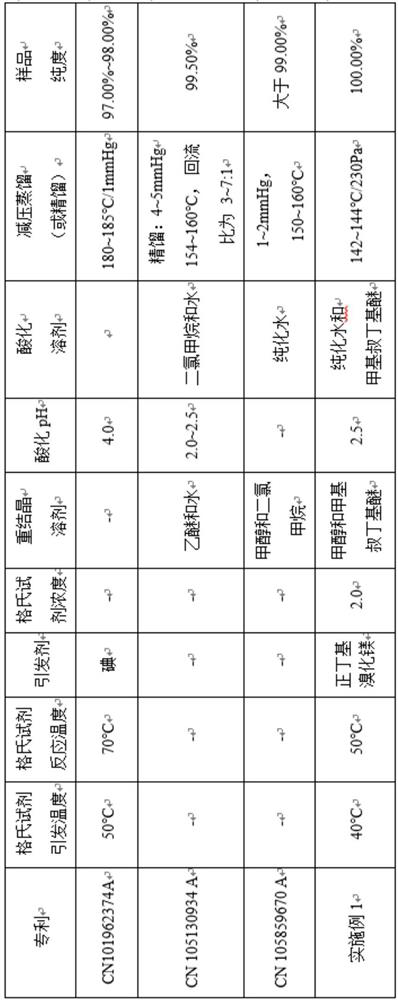
[0036] The HPLC described in the examples is measured by the area normalization method.
Embodiment 1
[0038] A preparation method of high-purity butylphthalide, comprising the steps of:
[0039] Step 1: Preparation of n-butylmagnesium bromide
[0040]Add 1.192 kg (49 mol) of magnesium chips and 10.2 kg (141.7 mol) of anhydrous tetrahydrofuran into the 50 L reactor, and replace with nitrogen three times under stirring, and then protect the reaction and post-treatment process under nitrogen gas. Stir and heat up so that the temperature in the reactor reaches 40°C and stabilizes. Stirring was stopped, and 350 mL of a mixed solution of n-butylmagnesium bromide and anhydrous tetrahydrofuran with a molar concentration of 2 mol / L was added to the reactor, and stirring was started and 6.4 kg (46.7 mol) of n-bromobutane and anhydrous tetrahydrofuran were added dropwise. The mixed solution of water tetrahydrofuran 6.4kg (88.9mol), observe that the internal temperature of the reactor rises, and the solution is produced with bubbles, indicating that the reaction is triggered, continue to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com