Carbazolyl-based organic double-heterocyclic near-infrared fluorescent probe as well as preparation method and application thereof
A fluorescent probe and double heterocycle technology, applied in the field of near-infrared fluorescent probes, can solve the problems of small Stokes shift and short emission wavelength of fluorescent probes, and achieve large Stokes shift and relatively long emission wavelength. The effect of long, simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] A method for preparing a carbazolyl-based organic biheterocyclic near-infrared fluorescent probe, the steps are as follows:
[0061] (1) Synthesis of 2,6-dimethyl-4-pyrylidene malononitrile 1
[0062] Put 2.48g (20mmol) of 2,6-dimethyl-4-pyrone and 1.59g (24mmol) of malononitrile in a 50ml single-necked flask, add 25ml of acetic anhydride, and heat the reaction at 142°C for 10 hours. The crude product was obtained by distillation under high pressure, and purified by silica gel chromatography column (solvent: mixed solvent of dichloromethane and methanol, methanol volume fraction 9-10%) to obtain the yellow-white solid product 2,6-dimethyl-4-pyranylidene Malononitrile, yield 86%.
[0063] The synthetic route is as follows:
[0064]
[0065] (2) Synthesis of 5-(9H-carbazol-9-yl)furan-2-carbaldehyde 2
[0066] in N 2 Under atmosphere, add 16mL N,N-dimethylformamide DMF, 2.006g (12mmol) carbazole and 2.65g (15mmol) 5-bromo-2-furfuraldehyde into a three-necked flask, ...
Embodiment 2
[0078] A method for preparing a carbazolyl-based organic biheterocyclic near-infrared fluorescent probe, the steps are as follows:
[0079] (1) The synthesis of 2,6-dimethyl-4-pyranylidene malononitrile 1 is the same as in Example 1;
[0080] (2) Synthesis of 5-(9H-carbazol-9-yl)thiophene-2-carbaldehyde 3
[0081] in N 2 Under the atmosphere, 16mL of DMF, 2.006g (12mmol) of carbazole and 2.87g (15mmol) of 5-bromothiophene-2-carbaldehyde were added into the three-necked flask, and stirred at room temperature for 30 minutes. Then add 0.38g (2mmol) cuprous iodide and 2.50g (18mmol) potassium carbonate, and react at 140°C for 48 hours. After the reaction, the solid was removed by filtration, and N,N-dimethylformamide was removed by a rotary evaporator. Purify by column chromatography (solvent: mixed solvent of n-hexane and ethyl acetate, ethyl acetate volume fraction 2-4%) to obtain pure product 5-(9H-carbazol-9-yl)thiophene-2-carbaldehyde, producing rate of 70%.
[0082] The...
experiment example
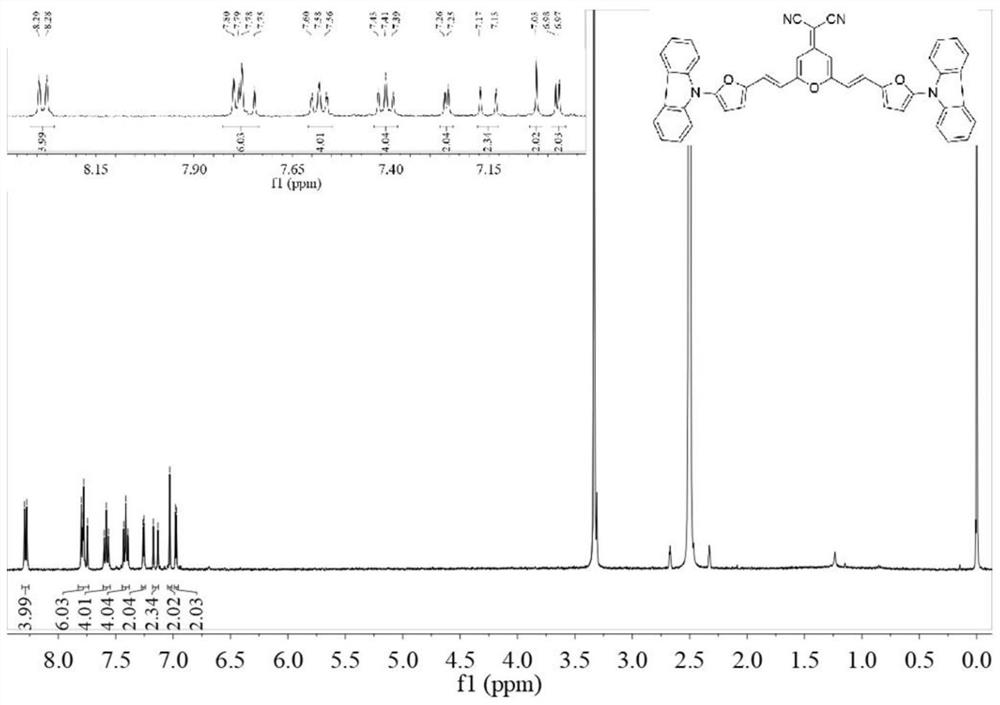
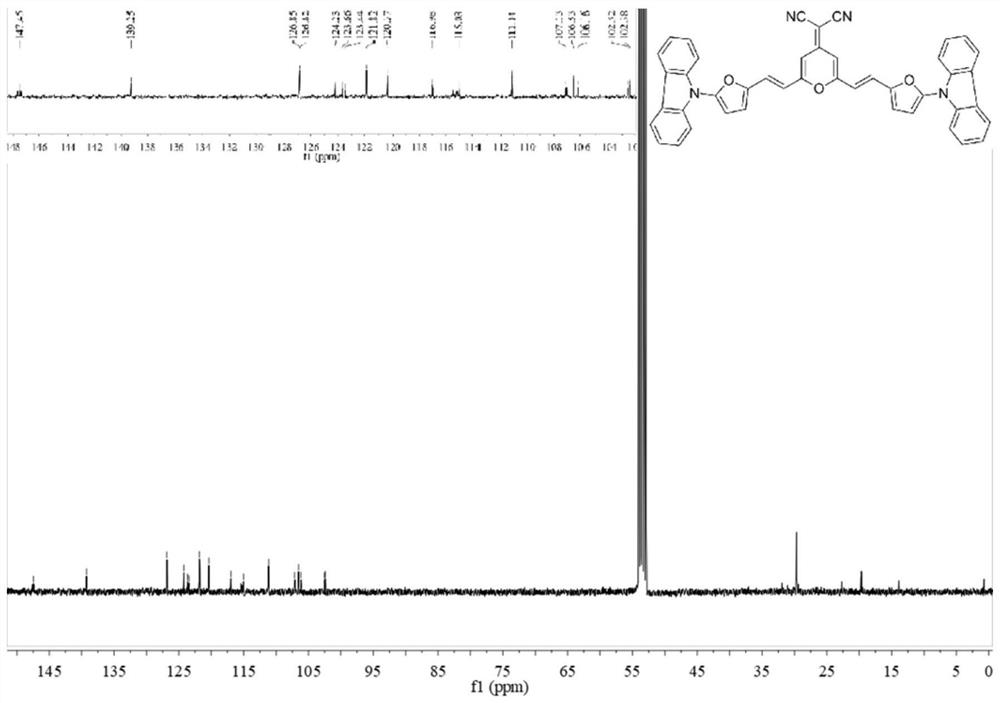
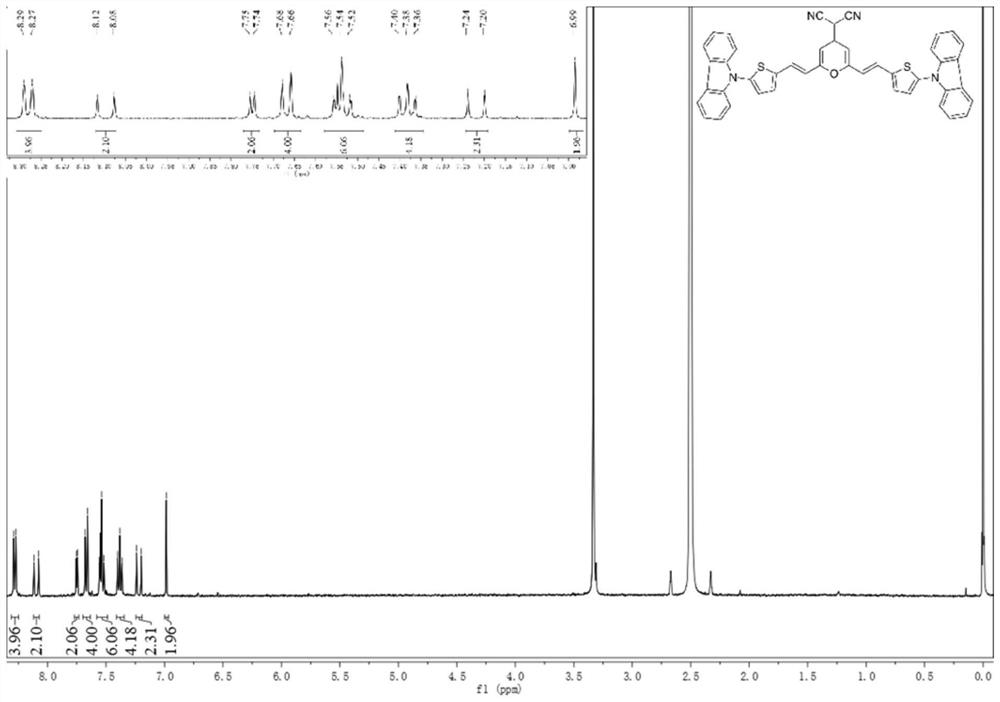
[0092] The near-infrared fluorescent probe II and near-infrared fluorescent probe III prepared above were subjected to liquid 1 H NMR and liquids 13 C NMR test, test results see respectively Figure 1-Figure 4 shown.
[0093]HITACHI F-7000 fluorescence spectrometer was used to test the fluorescence characteristics of the above-prepared near-infrared fluorescent probe II and near-infrared fluorescent probe III. The test results are shown in Figure 5-Figure 8 shown.
[0094] Test Results:
[0095] The excitation wavelength of the near-infrared fluorescent probe II is 465nm, the emission wavelength is 716nm, and the Stokes shift is 182nm. The excitation wavelength of the near-infrared fluorescent probe III is 455nm, the emission wavelength is 651nm, and the Stokes shift is 196nm; therefore, the near-infrared fluorescent probe of the present invention has a large Stokes shift and a long emission wavelength.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com