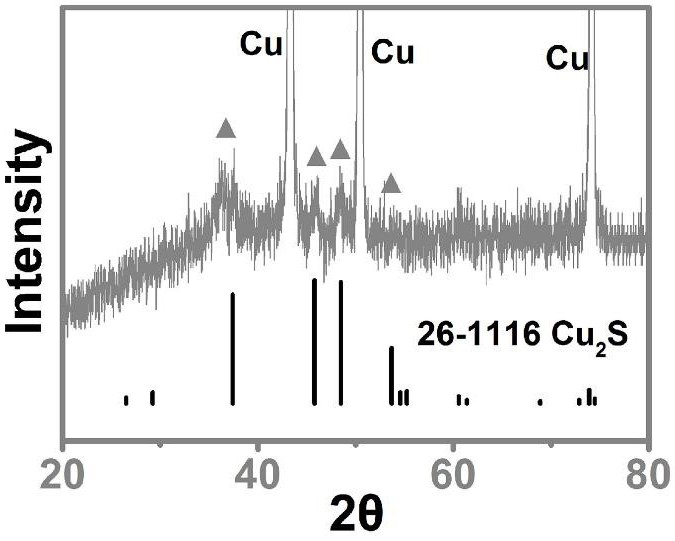
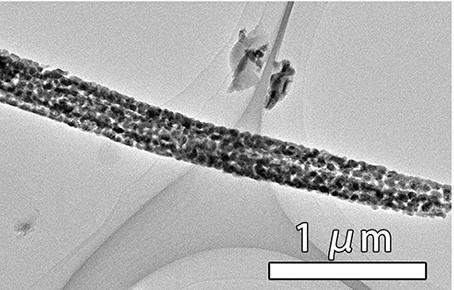
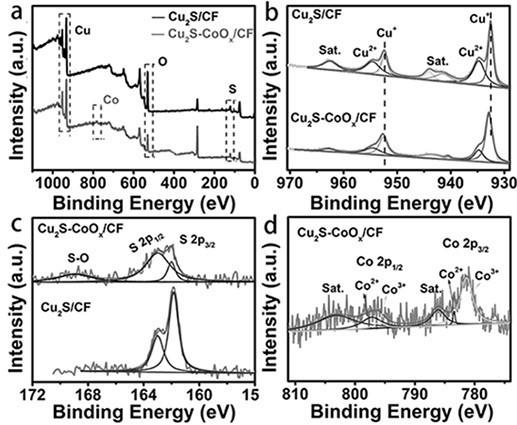
Preparation method of cuprous sulfide nanowire array efficient oxygen evolution catalyst of interface
A technology of nanowire array and cuprous sulfide, which is applied in the field of preparation of electrocatalytic oxygen evolution materials, can solve the problems of low intrinsic catalytic activity and poor surface electrochemical properties, so as to help electron transport and catalyze oxygen evolution Improvement of performance and improvement of electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0030] (1).NaOH (2 g) and (NH 4 ) 2 S 2 o 8 (456 mg) dissolved in 20 mL of deionized water, cut a piece of 2 × 3 cm 2 Copper foam (CF) was pretreated with 3 M dilute hydrochloric acid, deionized water and absolute ethanol for 15 min by ultrasonication;
[0031] (2).Reaction at room temperature: immerse the treated CF in step (1) into the solution prepared in step (1), and let it react at room temperature for 20 min;
[0032] (3). Washing and drying: After the reaction in step (2) was completed, the CF covered with the blue substance was taken out, washed with deionized water and absolute ethanol for 3 times, and then vacuum-dried at 60 °C for 6 h. Produce Cu(OH) 2 / CF Precursor;
[0033] (4). Preparation: Na 2 S·9H 2 O (180 mg) was sonicated in 20 mL of absolute ethanol;
[0034] (5). Ion exchange reaction: the Cu(OH) prepared in step (3) 2 / CF precursor was immersed in the solution in step (4), and allowed to react at room temperature for 2 h;
[0035] (6). Washing...
example 2
[0040] (1).NaOH (2 g) and (NH 4 ) 2 S 2 o 8 (456 mg) dissolved in 20 mL of deionized water, cut a piece of 2 × 3 cm 2 Copper foam (CF) was pretreated with 3 M dilute hydrochloric acid, deionized water and absolute ethanol for 15 min by ultrasonication;
[0041] (2).Reaction at room temperature: immerse the treated CF in step (1) into the solution prepared in step (1), and let it react at room temperature for 20 min;
[0042] (3). Washing and drying: After the reaction in step (2) was completed, the CF covered with the blue substance was taken out, washed with deionized water and absolute ethanol for 3 times, and then vacuum-dried at 60 °C for 6 h. Produce Cu(OH) 2 / CF Precursor;
[0043] (4). Preparation: Na 2 S·9H 2 O (180 mg) was sonicated in 20 mL of absolute ethanol;
[0044] (5). Ion exchange reaction: the Cu(OH) prepared in step (3) 2 / CF precursor was immersed in the solution in step (4), and allowed to react at room temperature for 2 h;
[0045] (6). Washing...
example 3
[0050] (1).NaOH (2 g) and (NH 4 ) 2 S 2 o 8 (456 mg) dissolved in 20 mL of deionized water, cut a piece of 2 × 3 cm 2 Copper foam (CF) was pretreated with 3 M dilute hydrochloric acid, deionized water and absolute ethanol for 15 min by ultrasonication;
[0051] (2).Reaction at room temperature: immerse the treated CF in step (1) into the solution prepared in step (1), and let it react at room temperature for 20 min;
[0052] (3). Washing and drying: After the reaction in step (2) was completed, the CF covered with the blue substance was taken out, washed with deionized water and absolute ethanol for 3 times, and then vacuum-dried at 60 °C for 6 h. Produce Cu(OH) 2 / CF Precursor;
[0053] (4). Preparation: Na 2 S·9H 2 O (180 mg) was sonicated in 20 mL of absolute ethanol;
[0054] (5). Ion exchange reaction: the Cu(OH) prepared in step (3) 2 / CF precursor was immersed in the solution in step (4), and allowed to react at room temperature for 2 h;
[0055] (6). Washing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com