Preparation of hydrazone chiral covalent organic framework material and application of hydrazone chiral covalent organic framework material in metal ion recognition
A technology of covalent organic framework and chirality, which is applied in the direction of material analysis, luminescent materials, and analytical materials through optical means, and achieves good application prospects, high stability, and good crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 A preparation method of hydrazone chiral covalent organic framework material
[0040] The preparation method comprises the following steps:
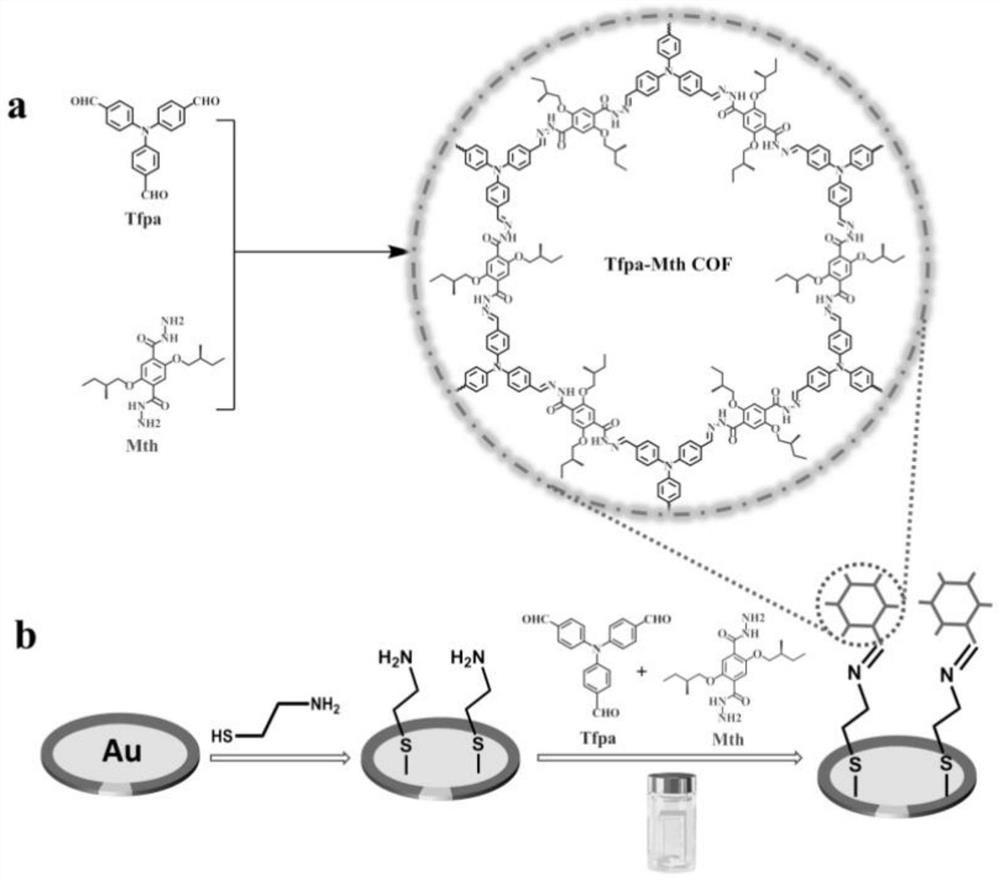
[0041] (1) Tris(4-formylphenyl)amine (Tfpa, 6.6mg, 0.02mmol), chiral hydrazide monomer (S)-2,5-bis(2-methylbutoxy) p-phenylene The mixture of dicarboxylic acid dihydrazide (Mth, 11.0mg, 0.03mmol) and 1.0mL of mesitylene solution was put into a 10mL pressure-resistant reaction flask, mixed thoroughly and then added with 0.1mL of 6M acetic acid solution.
[0042] (2) Bubble the pressure-resistant reaction vial with argon gas for 10 minutes, then quickly seal it, and then place the pressure-resistant reaction vial in an oven at 120° C. to react for three days.
[0043] (3) Cool to room temperature after the reaction, collect the precipitate by suction filtration, wash the precipitate with 1,4-dioxane, tetrahydrofuran and absolute ethanol several times in sequence, and finally put it in a vacuum drying oven for 24 hours at ...
Embodiment 2
[0044] The preparation method of embodiment 2COF-based QCM wafer
[0045] The preparation method comprises the following steps:
[0046](1) Soak the QCM chip in piranha solution (98% H 2 SO 4 :30%H 2 o 2 =7:3) for 10min, then rinse with a large amount of deionized water, dry with nitrogen, then soak the cleaned QCM chip in β-mercaptoethylamine solution for 12h, then rinse with deionized water, and dry with nitrogen to obtain Amino-modified QCM chips are ready for use.
[0047] (2) First put tris(4-formylphenyl)amine (Tfpa, 6.6mg, 0.02mmol) and mesitylene (8mL) into the reaction bottle for ultrasonic dissolution, and then put the above-mentioned amino-modified QCM chip face down Fix it on a polytetrafluoroethylene bracket and put it into the reaction bottle, then add the chiral hydrazide monomer (S)-2,5-bis(2-methylbutoxy)terephthalic acid dihydrazide (Mth , 11.0mg, 0.03mmol) and 0.2mL 6M acetic acid solution, the resulting mixture was bubbled with argon, then the reactio...
experiment example 1
[0048] Experimental Example 1 Performance Determination of Hydrazone Chiral Covalent Organic Framework Materials
[0049] The hydrazone chiral covalent organic framework material prepared in Example 1 was used as the material, and its performance was measured.
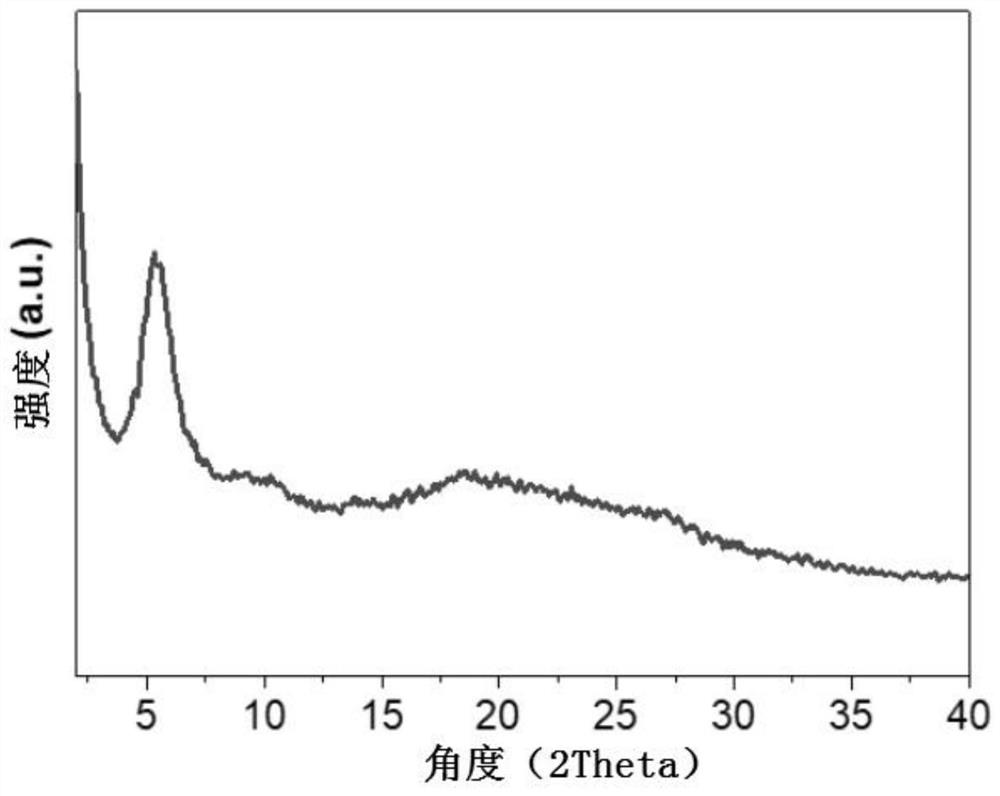
[0050] (1) X-ray powder diffraction measurement
[0051] The powder X-ray diffraction spectrum of the chiral covalent organic framework material prepared in Example 1 was measured on a Science Ultima IV X-ray powder diffractometer in Japan, using CuKα radiation, at a 2θ range of 2°-40°, at room temperature. Such as figure 2 As shown, 5.5° and 9.0° in the figure are the characteristic diffraction peaks of chiral covalent organic framework materials, indicating that it has better crystallinity.
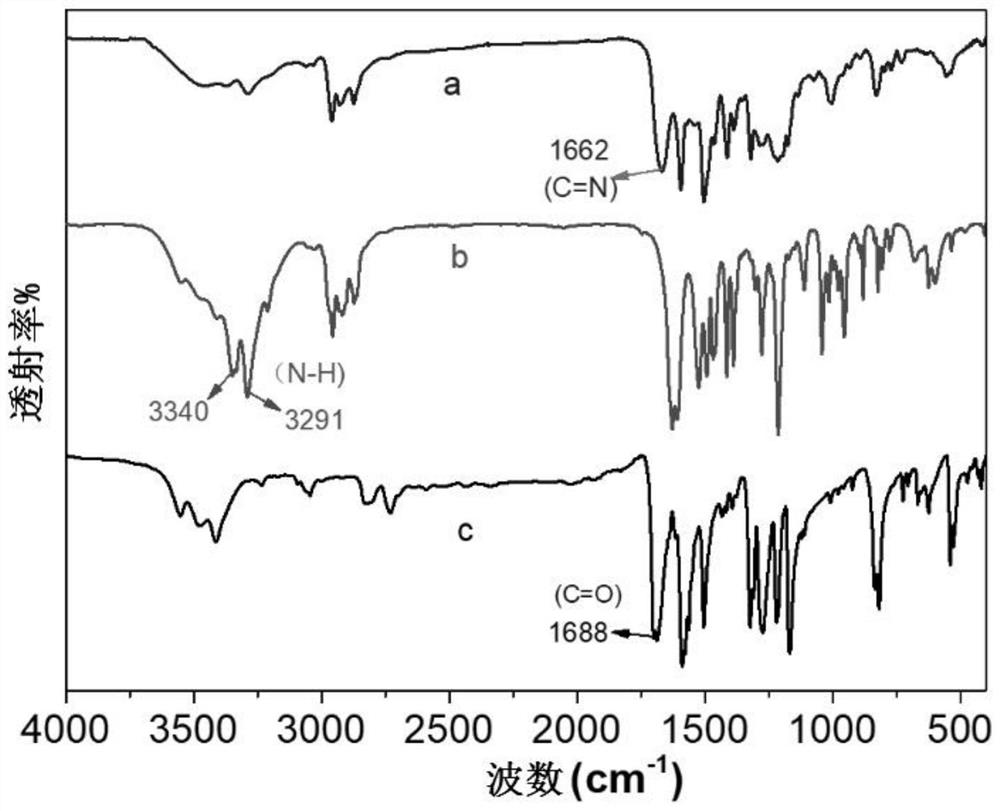
[0052] (2) Measurement of Fourier transform infrared spectroscopy
[0053] The chiral covalent organic framework material prepared in Example 1 was measured by Fourier Transform Infrared Spectroscopy (FT-IR). The determinati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com