Method for delivering exogenous molecules into T cells
An exogenous and molecular technology, applied in the field of biomedicine and medical devices, can solve the problems of unclear gene insertion site tumorigenic risk, low delivery efficiency, high cytotoxicity, etc., to improve cell harvest rate and delivery efficiency , the effect of a large number of cell treatments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035]
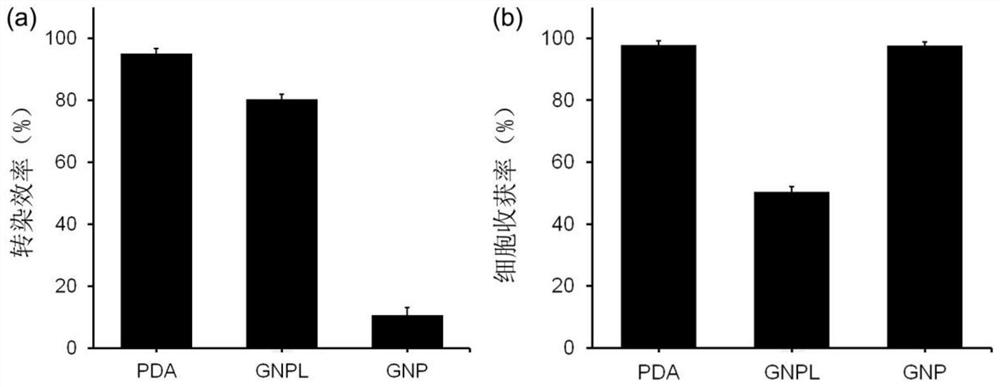
[0036] In the present invention, a photothermal transfer substrate is firstly prepared, and a polydopamine layer is deposited on its surface. The substrate with the polydopamine layer deposited on the surface of the present invention can be used to deliver exogenous molecules into T cells.
[0037] Dopamine (dopamine, DA, chemical structural formula: C 6 h 3 (OH) 2-CH 2 -CH 2 -NH 2 , CAS No.: 62-31-7) As one of the catechol derivatives, it is a neurotransmitter, a chemical substance used to help cells transmit pulses. In recent years, dopamine has been widely used in biomedicine and biomaterials because of its strong adhesion to the surface of substrates. Dopamine is easily oxidized by dissolved oxygen in aqueous solution, and then triggers a self-polymerization cross-linking reaction, which can form a closely adhered polydopamine composite layer on the surface of almost any solid material. Polydopamine is a melanin-like substance. It not only has good adhesi...
Embodiment 1
[0056] Step 1: Preparation of CAR-T cells
[0057] (1) Dissolve 0.08 g of dopamine hydrochloride in 40 mL of deionized water, and adjust the pH value to 8.5 with alkaline solution. Immerse the gold flakes in the above solution and let stand at 37° C. for 24 hours to obtain a substrate deposited with a polydopamine deposition layer.
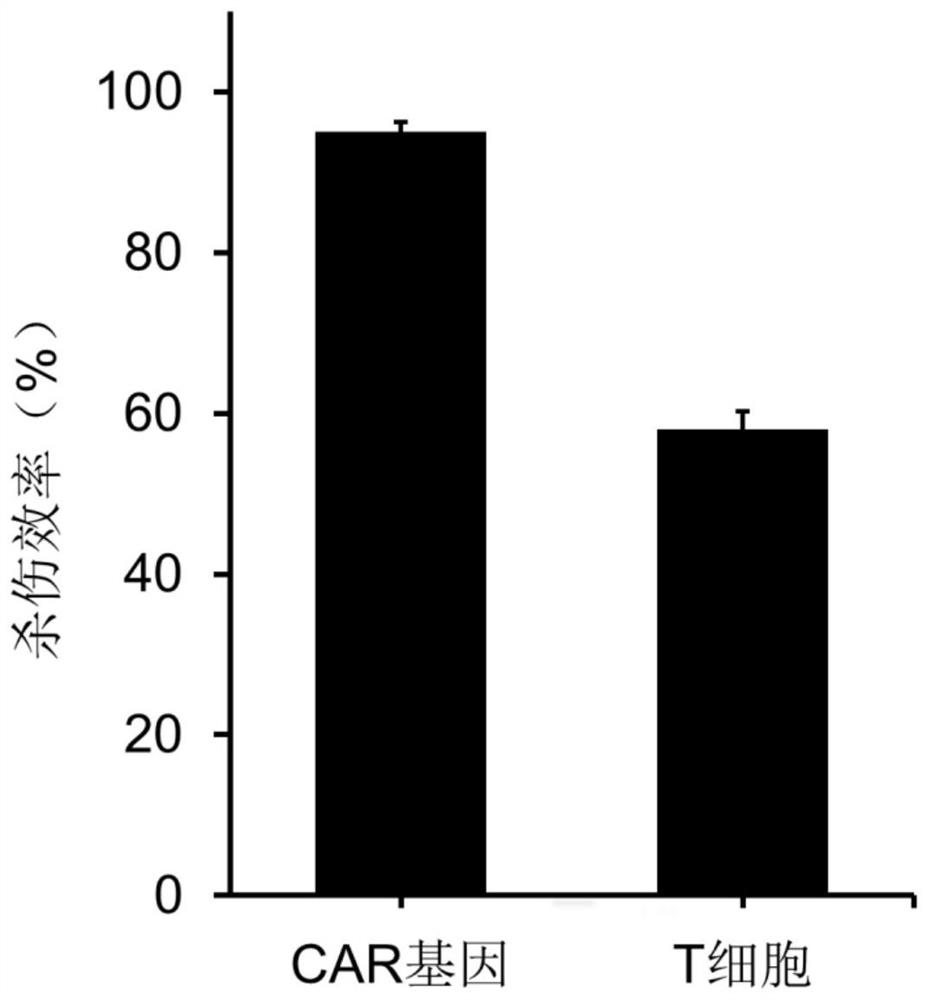
[0058] (2) Mix T cells and CAR gene (plasmid DNA encoding CAR protein, Lenti-EF1a-CD19(FMC63)-2nd-CAR(4-1BB)-EGFRt, Aikangde Bio) in a certain ratio in serum-free In the cell culture medium, the density of T cells is 200,000 / cm 2 , wherein the final concentration of the CAR gene was 0.006 μg / mL.
[0059] (3) The photothermal substrate is sterilized with 75% ethanol, and the above cell suspension is dropped onto the surface of the photothermal substrate to form a micron-sized liquid thin layer.
[0060] (4) Use a laser light source in the near-infrared band at 1W / cm 2 The cells on the sample were irradiated within the power density range for 3 ...
Embodiment 2
[0074] The CAR gene in Step 1 (2) of Example 1 was replaced with rhodamine-labeled dextran (molecular weight 4.4kDa), and Step 1 (1), (3)-(5) and Step 2 were the same as in Example 1.
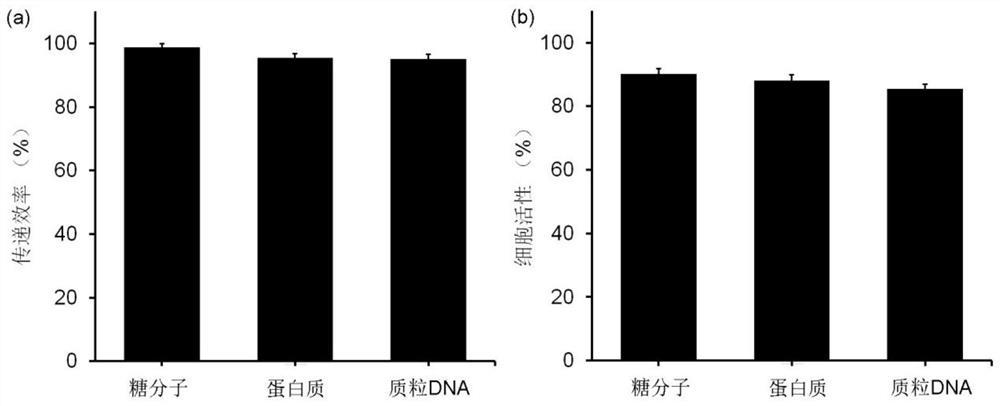
[0075] Replace (6) in Step 1 of Example 1 with: after 30 minutes of laser irradiation, stain the cell nucleus with 4',6-diamidino-2-phenylindole, and observe the entry of dextran into the cell with a fluorescence microscope . Cells in blue are stained nuclei, and red is the color emitted by rhodamine-labeled dextran, representing successfully delivered cells. The transfer efficiency was obtained by quantifying the fluorescence micrographs. In addition, the cell viability was detected by CCK-8 48 hours after laser irradiation. For delivery efficiency and cell viability histograms, see figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com