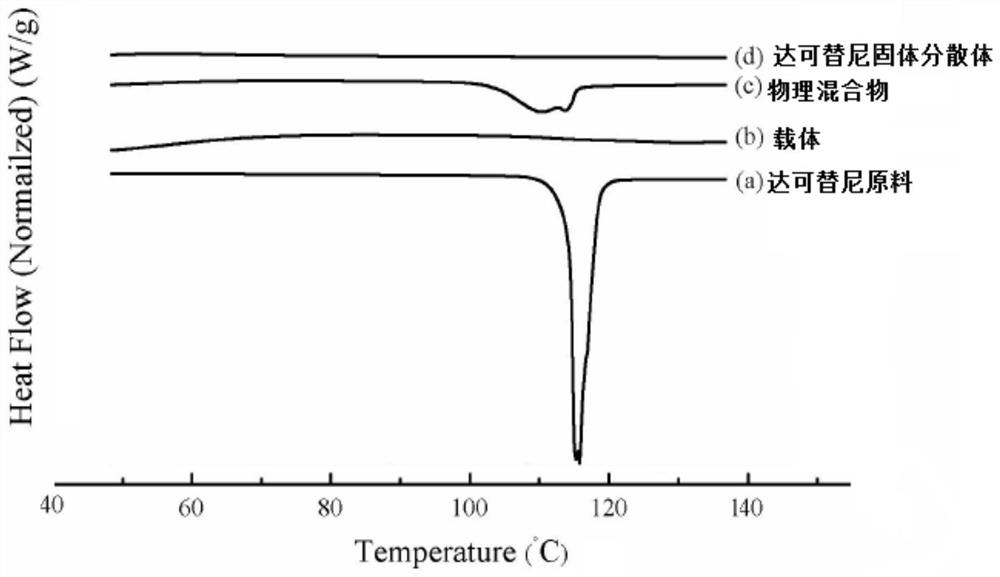
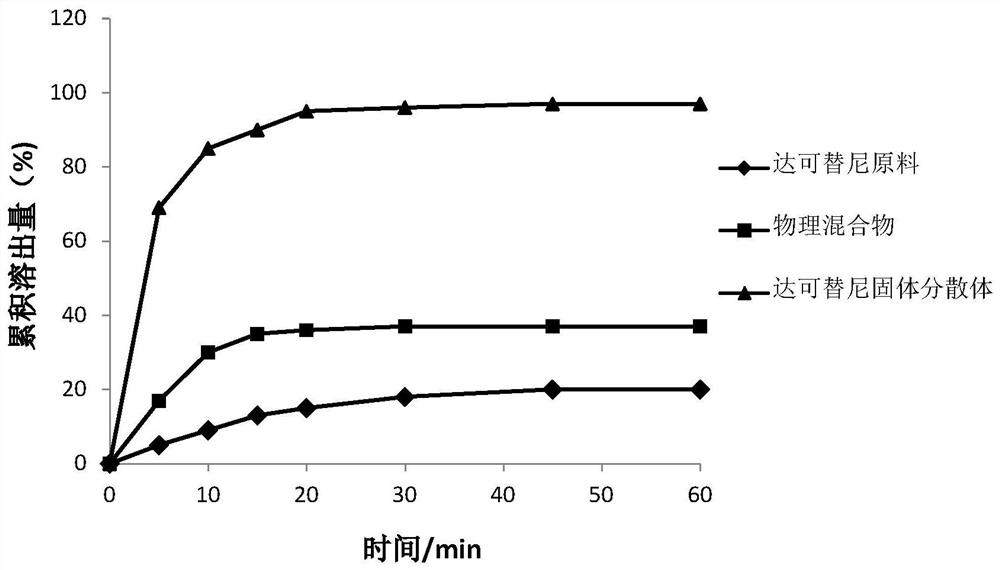
Dacomitinib quick-release preparation and preparation method thereof
A kind of preparation, Nisu’s technology, which is applied in the field of dacomitinib immediate-release preparations and its preparation, can solve the problems of poor dissolution of dacomitinib, and achieve the effects of improved in vivo and in vitro performance, high degree of automation, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A dacomitinib immediate-release preparation is made of the following components by mass percentage:
[0034]
[0035] Preparation:
[0036] (1) Pass dacomitinib, mannitol and hypromellose phthalate through an 80-mesh sieve and mix with a three-dimensional mixer to obtain a physical mixture;
[0037] (2) Set the hot-melt extrusion temperature to 120°C, and the screw speed to 50r / min. After the temperature reaches the set temperature, add the physical mixture evenly into the filler hopper of the hot-melt extruder, and pass through the hot-melt extruder The screw melts, mixes, and extrudes to obtain a strip-shaped extrudate;
[0038] (3) After the strip-shaped extrudate is cooled to room temperature, it is pulverized into particles with a pulverizer, and the particles pass through a 60-mesh sieve to obtain a solid dispersion of Dacomitinib;
[0039] (4) After mixing the solid dispersion of Dacomitinib with lactose, microcrystalline cellulose, sodium starch glycolate a...
Embodiment 2
[0044] A dacomitinib immediate-release preparation is made of the following components by mass percentage:
[0045]
[0046] Preparation:
[0047] (1) Pass dacomitinib, mannitol and hypromellose phthalate through an 80-mesh sieve and mix with a three-dimensional mixer to obtain a physical mixture;
[0048] (2) Set the hot-melt extrusion temperature to 120°C, and the screw speed to 50r / min. After the temperature reaches the set temperature, add the physical mixture evenly into the filler hopper of the hot-melt extruder, and pass through the hot-melt extruder The screw melts, mixes, and extrudes to obtain a strip-shaped extrudate;
[0049] (3) After the strip-shaped extrudate is cooled to room temperature, it is pulverized into particles with a pulverizer, and the particles pass through a 60-mesh sieve to obtain a solid dispersion of Dacomitinib;
[0050] (4) After mixing the solid dispersion of Dacomitinib with lactose, microcrystalline cellulose, sodium starch glycolate a...
Embodiment 3
[0053] A dacomitinib immediate-release preparation is made of the following components by mass percentage:
[0054]
[0055] Preparation:
[0056] (1) Pass dacomitinib, mannitol and hypromellose acetate phthalate through an 80-mesh sieve and mix with a three-dimensional mixer to obtain a physical mixture;
[0057] (2) Set the hot-melt extrusion temperature to 120°C, and the screw speed to 50r / min. After the temperature reaches the set temperature, add the physical mixture evenly into the filler hopper of the hot-melt extruder, and pass through the hot-melt extruder The screw melts, mixes, and extrudes to obtain a strip-shaped extrudate;
[0058] (3) After the strip-shaped extrudate is cooled to room temperature, it is pulverized into particles with a pulverizer, and the particles pass through a 60-mesh sieve to obtain a solid dispersion of Dacomitinib;
[0059] (4) After mixing the solid dispersion of Dacomitinib with lactose, microcrystalline cellulose, sodium starch glyco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com