Bridged asymmetric benzodiazole and/or pyridine diazole double-acceptor polymer semiconductor, preparation method and application thereof
A technology of benzodiazoles and pyridinediazoles, applied in the field of polymer semiconductor materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] A kind of polymer semiconductor material whose chemical structure is PBT-fBTT, its synthetic route is as follows:
[0098]
[0099] (1) the chemical structure formula is the synthesis of the intermediate of a: under nitrogen protection, add tributyl-[4-(2-decyltetradecyl) thiophen-2-yl] alkyl tin (Suzhou Na Kai Technology Co., Ltd.) (5.8g, 8.2mmol), 4,7-dibromo-2,1,3-benzothiadiazole (2.0g, 6.8mmol), bis(triphenylphosphine) dichloride Palladium (0.24g, 0.34mmol) catalyst, 70mL toluene solvent. After reflux for 4 hours, cool to room temperature. Using dichloromethane for extraction, the organic phase was dried with magnesium sulfate, and the solvent was spin-dried to obtain a crude product. Then adopt silica gel chromatographic column purification to obtain the target product 4-bromo-7-(4-(2-decyltetradecyl)thiophen-2-yl)-benzene[c][1,2,5]thiazole, the yield = 85%.
[0100] The structural characterization data are as follows,
[0101] 1 H NMR (400MHz, CDCl 3 ),...
Embodiment 2
[0127] A kind of polymer semiconductor material whose chemical structure is PBT-PTT, its synthetic route is as follows:
[0128]
[0129] (1) Synthesis of an intermediate whose chemical structural formula is a: Synthesize with reference to the synthetic method of the above-mentioned Example 1.
[0130] (2) Synthesis of an intermediate whose chemical structural formula is b: under nitrogen protection, tributyl-[4-(2-decyltetradecyl)thiophen-2-yl]alkyltin (5.8g , 8.2mmol), 4,7-dibromo-[1,2,5]thiadiazolo[3,4-c]pyridine (2.0g, 6.8mmol), bis(triphenylphosphine)palladium dichloride (0.24g, 0.34mmol) catalyst, 70mL toluene solvent. After reflux for 4 hours, cool to room temperature. Using dichloromethane for extraction, the organic phase was dried with magnesium sulfate, and the solvent was spin-dried to obtain a crude product. Then use silica gel column purification to obtain the target product 7-bromo-4-(4-(2-decyltetradecyl)thiophen-2-yl)-[1,2,5]thiazole[3,4-c] Pyridine, yi...
Embodiment 1 and 2
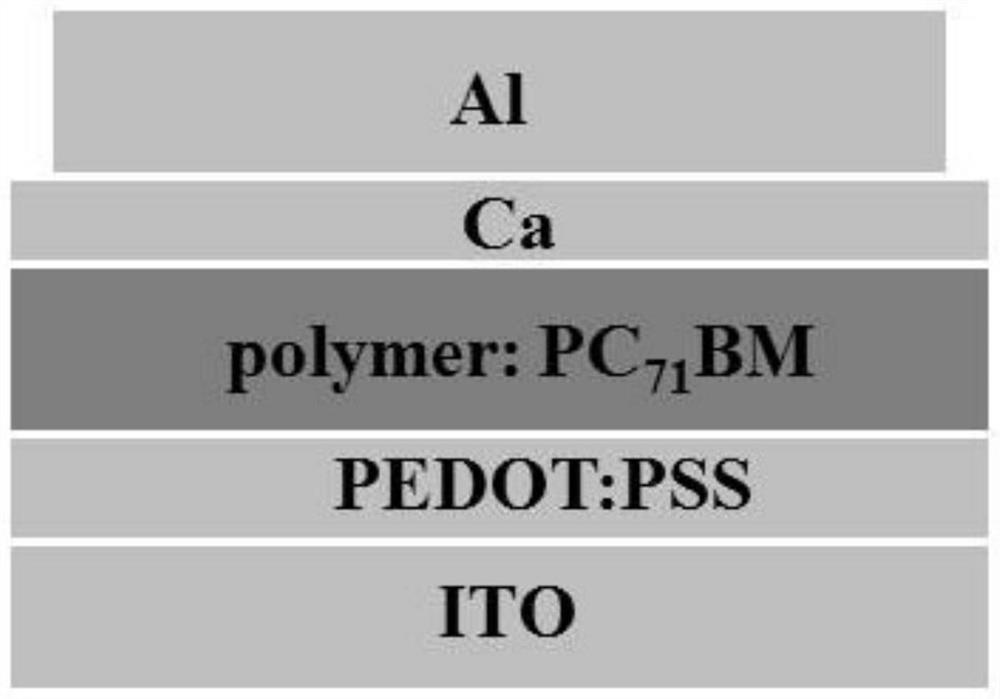
[0152] The measurement of the spectroscopic properties of the polymer PBT-fBTT and PBT-PTT and polymer solar cell and organic photodetector properties prepared by above-mentioned embodiment 1 and 2:
[0153] (1) Absorption spectral properties of polymers PBT-fBTT and PBT-PTT
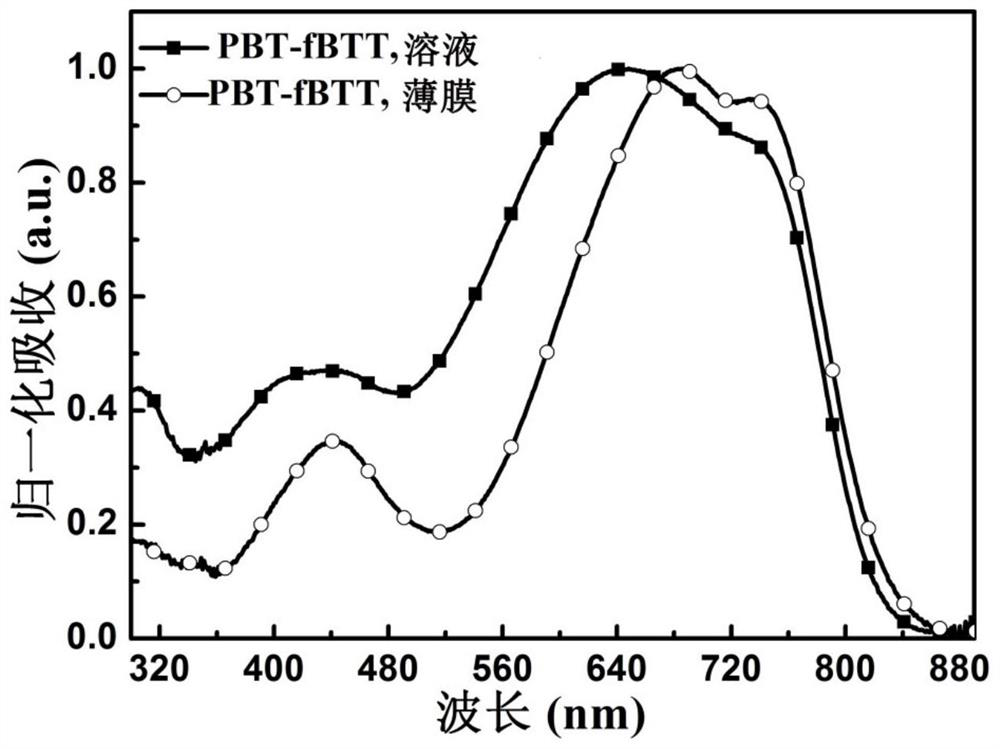
[0154] figure 1 UV-visible-near-infrared absorption spectra of polymer PBT-fBTT in chlorobenzene solution and thin film on quartz wafer. Depend on figure 1 It can be seen that both the polymer PBT-fBTT solution and the film exhibit a wide absorption range, and the maximum absorption sideband value of the film is about 835nm, and the corresponding optical band gap is 1.49eV (the optical band gap is calculated according to the formula E g =1240 / λ calculation, where E g is the optical bandgap, and λ is the maximum absorption sideband value of film absorption).
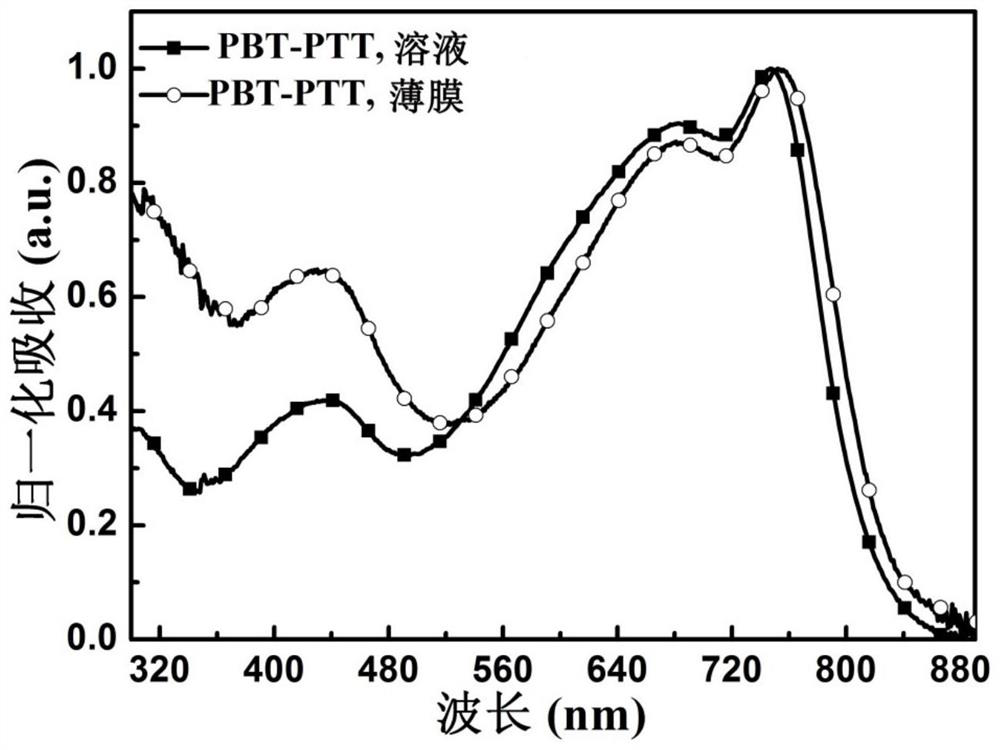
[0155] figure 2 UV-visible-near-infrared absorption spectra of polymer PBT-PTT in chlorobenzene solution and thin film on quartz wafer. Depe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com