Tyrosinase-activated double-quenching diagnosis and treatment prodrug and preparation thereof
A tyrosinase and quenching technology, which is applied in the field of double-quenched fluorescence diagnostic prodrug compounds, can solve the problems of low fluorescence masking efficiency and incomplete transfer, and achieve high drug loading, high activity and high response rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
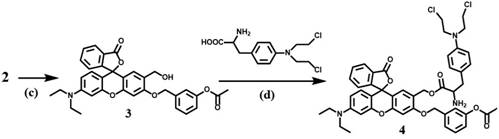
[0026] A method for preparing a tyrosinase-activated double-quenching prodrug for diagnosis and treatment, the steps comprising:
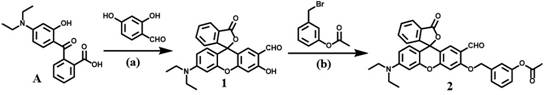
[0027] 1) Synthesis of Compound 1:
[0028] To prepare compound A from phthalic anhydride and 3-diethylaminophenol, compound A (3.15 g, 10 mmol) and 2,4-dihydroxybenzaldehyde (2 g, 12 mmol) were dissolved in trifluoroacetic acid solution (25 mL), stirred at 80°C for 18 hours, poured the cooled mixture into 25 mL of ice water, and washed with saturated Na 2 CO 3 The aqueous solution was neutralized to a pH of 7.5, the solvent was evaporated under reduced pressure, and column purification (dichloromethane / ethanol=20 / 1) gave compound 1 as a red powder with a yield of 50%.
[0029] Synthesis of Compound 2:
[0030] Compound 1 (0.75 g, 1.8 mmol), methyl 3-(bromomethyl)benzoate (0.62 g, 2.7 mmol) and Cs 2 CO 3 (1.05 g, 3.24 mmol) was dissolved in DMF solution (15 mL), stirred at 75 °C for 15 hours, after cooling to room temperature, 25 mL of water w...
Embodiment 2
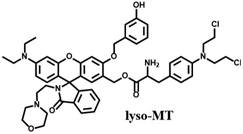
[0038] In Vitro Detection Method of Reaction of Prodrug Molecule lyso-MT with Tyrosinase
[0039]In a test tube, take the prodrug molecule lyso-MT (50 μM) synthesized in Example 1 and mix thoroughly in 4 mL phosphate buffer saline and DMSO to prepare a stock solution, then add tyrosinase solution, and phosphate buffer saline The volume was adjusted to 5 mL. After reacting at 37°C for 30 minutes in the incubator, transfer 3 mL of the reaction solution to a 1 cm quartz cell, and ex / em Fluorescence is measured at wavelengths = 470 / 528nm. At the same time, a solution without adding tyrosinase was prepared as a control, and compared under the same conditions.
Embodiment 3
[0041] Fluorescent response of prodrug molecule lyso-MT to tyrosinase
[0042] Taking the prodrug compound solution (10 μM) in Example 2, after adding tyrosinase, it was observed that the fluorescence at about 528 nm increased by about 55 times, and the fluorescence quantum yield increased from less than 0.01 to 0.14. lyso-MT exhibited a good linear fluorescence response to tyrosinase in the concentration range from 0.5 to 60 U / mL, and the detection limit was determined to be 0.7 U / L, indicating that the prodrug has a sensitive and rapid response to tyrosinase .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com